Research
Carriers of the Solute Carrier Family SLC10
Solute carriers (SLC) represent a group of membrane-bound transporter proteins, including more than 400 single carriers organized into 65 families. Among them, solute carrier family SLC10 includes seven members, i.e. SLC10A1-SLC10A7. The hepatic Na+/taurocholate cotransporting polypeptide NTCP (gene symbol SLC10A1) and the ileal apical sodium-dependent bile acid transporter ASBT (gene symbol SLC10A2) represent bile acid carriers responsible for the enterohepatic circulation of bile acids between the liver and the gut.
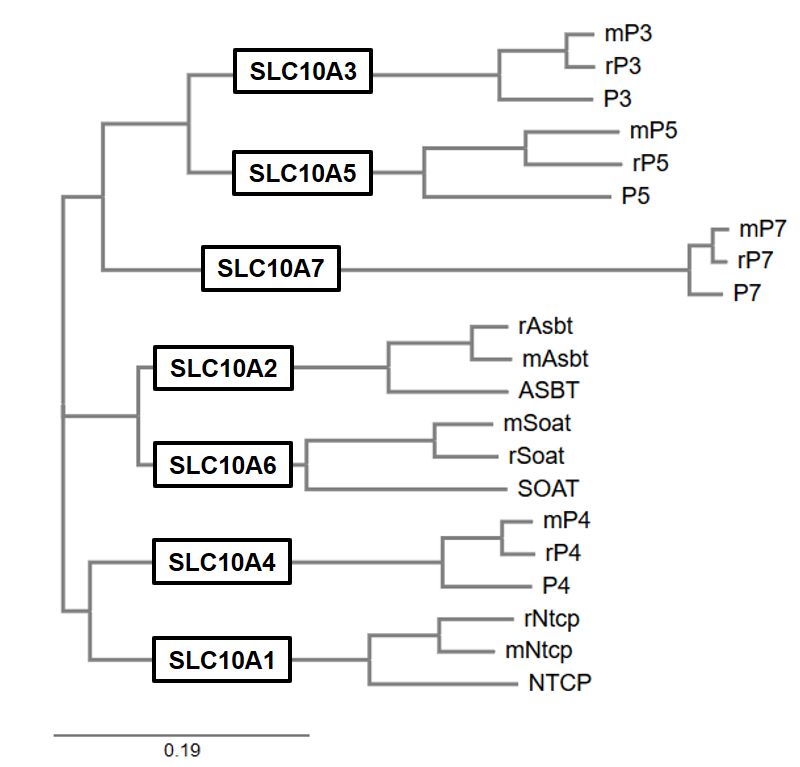
Phylogenetic tree of the SLC10 carriers SLC10A1-SLC10A7 from man (capital letters), rat (prefix “r”) and mouse (prefix “m”). Note that the SLC10A2/SLC10A6, SLC10A1/SLC10A4, and SLC10A3/SLC10A5 gene pairs each derive from common ancestors.
Apart from that, NTCP was recently identified as the high-affinity liver receptor for the hepatitis B (HBV) and hepatitis D (HDV) viruses and, therefore is an attractive drug target for HBV/HDV entry blockade (see below). However, not all SLC10 carriers are bile acid carriers, as the sodium-dependent organic anion transporter SOAT (gene symbol SLC10A6) represents a specific carrier for sulfated steroid hormones, expressed in reproductive organs and breast cancer. In the case of SLC10A3, SLC10A4, SLC10A5 and SLC10A7, the transport function could not be elucidated so far. Thus, they are orphan carriers.
Deorphanizing SLC10A3, SLC10A4, SLC10A5, and SLC10A7. By use of newly developed antibodies, stably transfected cell lines, and knockout mice, we aim to characterize the expression, regulation and transport function of these supposed carriers in order to elucidate their physiological and pharmacological relevance.
SLC10A4 is expressed in synaptic vesicles of cholinergic and monoaminergic neurons of the central and peripheral nervous system and here most likely is involved in the vesicular accumulation of neurotransmitters or their release by vesicle exocytosis. SLC10A5 is involved in the bile acid homeostasis and is expressed in so far unidentified vesicular compartments in the liver and kidneys. SLC10A7 represents a negative regulator of the calcium influx and plays a role for protein glycosylation and proper skeletal development.
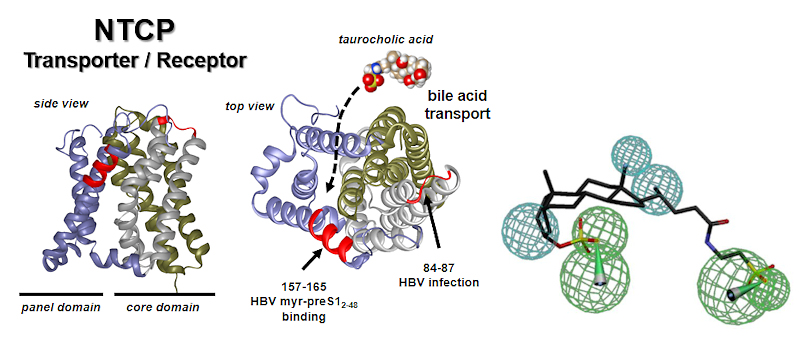
Development of HBV/HDV entry inhibitor by blocking of the transporter/receptor NTCP. HBV/HDV attach via their myristoylated preS1 (myr-preS1) peptide domain of the large surface protein to NTCP, representing the initiating step of HBV/HDV infection. We use pharmacophore modelling (as exemplified in the right panel) to identify NTCP inhibitors which specifically block HBV/HDV virus binding to NTCP, but without blocking the bile acid transport function of NTCP.
Furthermore, we are searching for specific inhibitors of NTCP and SOAT by pharmacophore modelling in order to prevent HBV/HDV entry into hepatocytes in the case of NTCP and to inhibit the uptake of pro-proliferative steroid precursors via SOAT in cancer cells.
MDR1 mutation in dogs and neurological toxicity of antiparasitic drugs
P-Glycoprotein (often referred to as MDR1) is an ATP-driven efflux transporter (ABC transporter), encoded by the multidrug-resistance gene MDR1/ABCB1. It transports a wide range of structurally unrelated lipophilic and amphipathic drugs and toxins, including many commonly used veterinary drugs. MDR1 is highly expressed at the blood-brain barrier, where it restricts the entry of drugs (such as ivermectin) and toxins into the central nervous system. In certain dog breeds, such as Collie, Australian Shepherd, Shetland Sheepdog, White Shepherd and others, an exonic 4-bp deletion can occur in the MDR1 gene (nt230(del4) MDR1 mutation). Dogs, homozygous for this mutation (MDR1-/-) are highly sensitive to many MDR1-transported drugs, among them many antiparasitic drugs such as ivermectin. Ivermectin toxicosis in affected MDR1-/- dogs includes depression, ataxia, somnolence, salivation, tremor, coma, and death already at therapeutic doses of 0.2 mg/kg. This can be prevented by MDR1 genotyping prior to drug treatment - MDR1 genotyping.
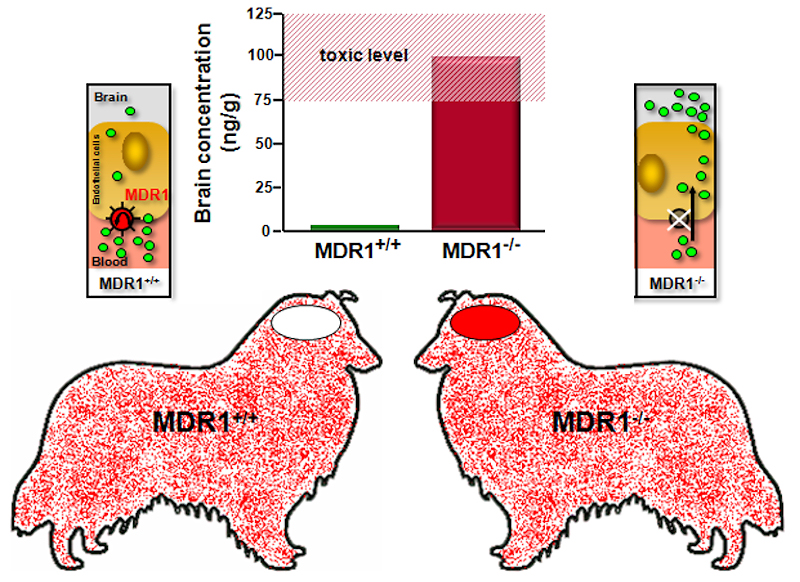
MDR1, expressed at the apical domain of the endothelial cells of the brain capillaries, highly restricts the entry of ivermectin (green spots) from the blood into the brain. Therefore, brain levels of ivermectin are low and so this drug can be safely used in dogs with intact MDR1 gene (MDR1+/+). In dogs with homozygous MDR1 deletion mutation (MDR1-/-), however, the barrier function of MDR1 is abolished and ivermectin can enter the brain, where it provokes severe and life-threatening neurological toxicity already at therapeutic dosage.
Neuropharmacology
The aim of our neuropharmacological research is to identify mechanisms that underlie the occurrence of different neurological diseases in people and animals as well as preclinical drug investigations. Predominantly, we are interested in movement disorders that are based in the central nervous system (CNS) like Morbus Parkinson and dystonia. Furthermore, our studies comprise disorders of learning and memory function like Morbus Alzheimer and other types of dementia. Cognitive dysfunctions are a challenge not only in human medicine. There is growing evidence that dogs and cats can also suffer from a “cognitive dysfunction syndrome”. The physiological, i. e. “normal”, neurological function depends on the precise adjustment of various neurotransmitter systems in the brain. Neurotransmitters represent chemical messengers that are used by the nervous system for the communication between different types of neurons. A neurotransmitter is able to influence a neuron in one of three ways: excitatory, inhibitory or modulatory. If one of the different neurotransmitter systems fails, there will be imbalances, which lead to neurological dysfunctions. We are mainly interested in three key neurotransmitters:
a) acetylcholine, which plays an important role in the central nervous system in maintaining cognitive function,
b) γ-aminobutyric acid (GABA), which represents the main inhibitory neurotransmitter in the CNS and
c) dopamine, which is involved in many functions, including motor control, reward and reinforcement, and motivation.
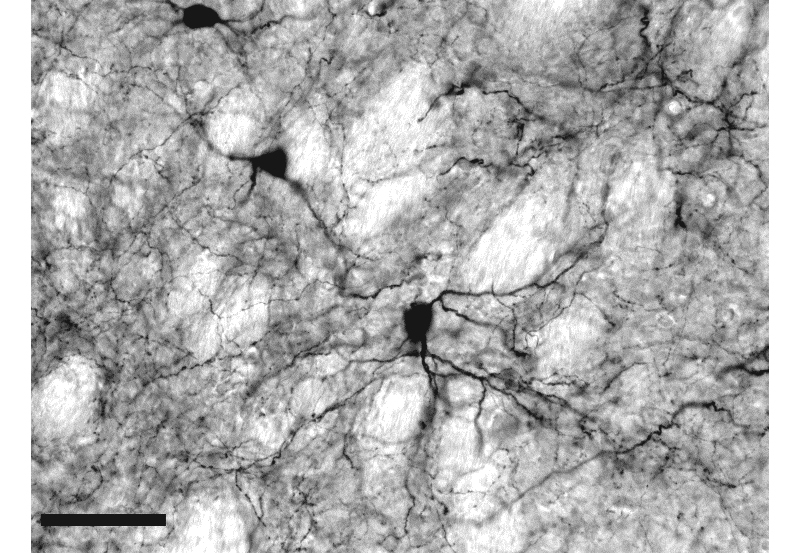
Photomicrographs of coronal sections illustrating a special type of GABAergic interneurons in the striatum, a brain structure that plays a critical role in the control of movement. Calibration bar: 50 µm.
Another research focus represents the topic neurotoxicology as already exemplarily mentioned in the chapter about MDR1 mutation in dogs. A wide variety of chemical substances and drugs are able to pass undesirably into the CNS, where they interact with neuronal structures, impair or damage their functions and therefore lead to neurological disorders. We are particularly interested in how and to what extent substances penetrate the blood-brain-barrier and pass into the CNS and how this transition can be modulated. Gender-specific aspects are included in all studies because steroids exert a multifaceted influence on the CNS. Meanwhile, it is known that specific “neurosteroids” mediate their actions in the CNS not only through classic steroid hormone nuclear receptors within several hours to days, but also in a paracrine fashion through fast acting mechanisms. Therefore, they can directly or indirectly modulate different neurotransmitter receptors.
