Kupfer(I) katalysierte Aminooxygenierung von Alkenen
Cu(I) catalyzed Aminooxygenation of alkenes
Nitrogen containing heterocycles, i.e. alkaloids, are often biologically active and some are used for highly interesting medicinal applications. Pyrrolidines and piperidines are building blocks frequently found in these biologically active molecules. There are various methods of synthesis for these nitrogen heterocycles, one of which is the transition metal catalyzed intramolecular radical aminooxygenation of alkenes.
The aminooxygenation of various N-Benzoyloxyamines by way of CuPF6 and BF3 was pioneered by R. Göttlich et. al.[1], and optimized to an extent.
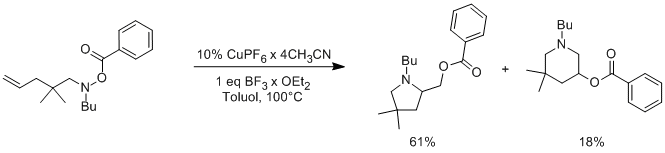
Currently we are investigating the effects of an EWG attached to the aromatic group on the N-O bond cleavage, as well as the steric effect of various substituents on the carbon chain forming the ring.
| Projekt director: | Prof. Dr. Richard Göttlich |
| Co-worker: |
[1] M. Noack, R. Göttlich, Chem. Commun., 2002, 536-537
