AG Tikkanen
AG Tikkanen develops enzyme replacement therapy for a rare disease. (March 9th, 2022) press release
AG Tikkanen discovers new mechanisms in autoimmune dermatosis therapy. (Oct 10th, 2024) Link: Frontiers
copyright: private
Rare Diseases Research Group
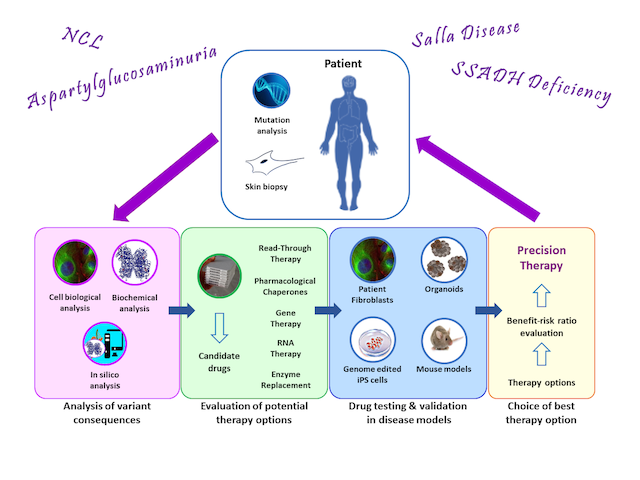
The main research interest of our group are the molecular mechanisms of rare diseases and development of personalized therapies for such diseases. The spectrum of diseases in our research focus include lysosomal storage disorders (aspartylglucosaminuria, neuronal ceroid lipofuscinoses), disorders of neurotransmitter metabolism (SSADH deficiency), and autoimmune diseases (Pemphigus vulgaris). In addition, we are interested in the molecular mechanisms of cancers and the role of flotillin proteins in cancer biology.
Press release (21.10.2021): Neuer Therapieansatz für genetische Erkrankungen
Aspartylglucosaminuria (R. Tikkanen and A. Banning)
Aspartylglucosaminuria (AGU) is a rare genetic disorder caused by mutations in the gene encoding for the lysosomal enzyme aspartylglucosaminidase (AGA). AGU patients are born seemingly normal, but within the first years of life, they start lagging behind in their development and become increasingly handicapped and intellectually disabled by early adulthood. Currently, no approved therapies are available for AGU.
Our group focuses on characterization of the molecular consequences of the AGU mutations, with the aim of developing individual therapies for AGU. Our approaches include pharmacological chaperones (PC) and nonsense read-through drugs, and we are also involved in developing gene therapy for AGU. So far, we have developed two PC therapies that are currently tested in patients (Banning et al. 2016). An investigator-initiated clinical trial with one of these substances (https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-000645-48/FI) is currently undergoing in Finland, together with Dr. Minna Laine, a child neurologist at Helsinki University Hospital. For patients exhibiting nonsense mutations combined with certain missense mutations, we have shown that substances that inhibit the nonsense-mediated decay of mRNA and induce a translational read-through of the nonsense codon are beneficial in AGU and result in increased enzyme activity in patient cells (Banning et al. 2018). One of these substances has also been tested in an individual clinical trial. Furthermore, we are developing small molecule treatment for gene variants that result in deficient splicing of the mRNA.
Gene therapy approach for AGU is currently being developed in collaboration with UTSW researchers, especially Dr. Steven Gray, and a US company (see: https://www.utsouthwestern.edu/labs/gray/). Gene therapy has recently been tested in AGU mouse model, showing evidence for clinical efficacy (Chen et al. 2021).
SSADH Deficiency (R. Tikkanen and M. Didiasova)
Succinyl semialdehyde dehydrogenase (SSADH) is a mitochondrial enzyme involved in the catabolism of the neurotransmitter GABA. SSADH deficiency (SSADH-D) is a genetic disorder caused by mutations in the gene encoding the SSADH enzyme. In the absence of SSADH activity, GABA and its metabolite GHB accumulate in the
tissues and cause mainly neuronal and muscular deficits. Our current research interests are the characterization of the molecular consequences of SSADH mutations and testing of various therapy options in cell culture models (see Didiasova et al. 2020, Brennenstuhl et al. 2020). As with AGU and NCLs, we are interested in identifying PC substances that would restore the missing SSADH activity, and probing for the read-through therapies. In addition, we are developing strategies suitable for enzyme replacement and gene therapy for SSADH-D.
Neuronal Ceroid Lipofuscinoses (R. Tikkanen)
Neuronal ceroid lipofuscinoses (NCL) are a group of lysosomal storage disorders that result in a severe developmental delay associated with vision loss and epileptic seizures that are difficult to control. Depending on the respective gene defect, these diseases show a different age of onset and disease progression and are classified into various forms (CLN1 - CLN14). Our group is interested in the classic late infantile (cLINCL, CLN2, CLN5) and juvenile (JNCL, CLN3) forms. Therapies are available for only very few NCL forms.
We have previously shown that specific membrane lipids termed gangliosides exhibit altered amounts in JNCL (Somogyi et al. 2018). Especially the ganglioside GM3 accumulates in high amounts, whereas other gangliosides such as GM1 are reduced. Since balanced amounts of various gangliosides are vital for normal brain development, we are currently testing if modulation of ganglioside amounts in NCLs might have a therapeutic effect.
Also in NCL diseases, we are interested in developing therapies that are based on similar strategies as described above for AGU, namely read-through therapies and pharmacological chaperones. Since certain nonsense mutations are very common especially in cLINCL patients, the read-through approach may provide a means to treat a large number of patients with the same drug. In addition, substances that affect splicing may also provide a potential therapy option for one of the most common cLINCL gene variants. In addition, we are involved in an industry-driven gene therapy project for CLN5.
copyright: private
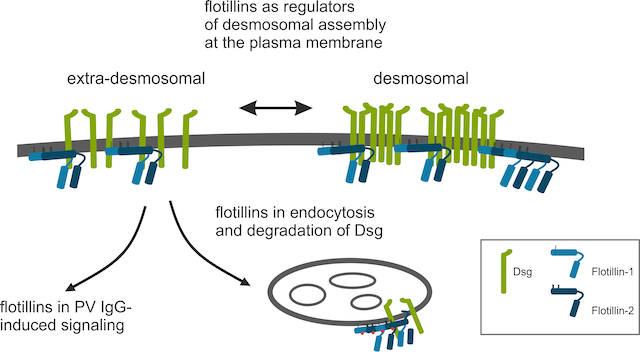
Function of Flotillin Proteins in Cell Adhesion: Molecular Mechanisms of Pemphigus Vulgaris (R. Tikkanen, A. Banning and A. Zakrzewicz)
Our current work includes the characterization of the molecular function of the flotillin family of proteins. Flotillins were originally described as neuronal regeneration proteins that are upregulated in the regenerating axons of gold fish retinal ganglion cells after a lesion of the optic nerve and thus named “reggies” for regeneration. Later studies have shown that flotillins are ubiquitously expressed, highly conserved and associated with membrane rafts. Our previous work has focused on characterization of flotillin function in signal transduction and cell adhesion. We have shown that flotillins are involved in both cell-matrix and cell-cell adhesion (Banning et al. 2018, Völlner et al. 2016). We could show that flotillins are involved in the regulation of desmosomal adhesion in the epidermis, and they interact with the desmosomal cadherin proteins and plakoglobin (Völlner et al. 2016).
Our current research focuses on elucidating the molecular mechanism of how desmosomal adhesion is regulated by flotillins, and how flotillins modulate the desmosomal adhesion in the severe autoimmune skin disease Pemphigus Vulgaris (PV). In PV, autoantibodies against desmosomal adhesion proteins, mainly desmoglein-3, cause severe blistering of the epidermis and mucosa due to loss of desmosomal adhesion in epidermal keratinocytes. The mechanisms of adhesion loss are as yet not completely characterized, but signaling and vesicle trafficking are likely to play a role. Flotillins are known to be involved in both signaling and membrane trafficking, and we are currently studying how flotillins modulate these processes in the context of Pemphigus. We are members in the DFG-funded Research Focus FOR 2497 “Pemphigus - from Pathogenesis to Therapy (Pegasus)”. (www.pegasus-dfg.de). In addition to their role in the regulation of desmosomes in the epidermis, we are also interested in how flotillins affect desmosomal adhesion in the cardiac tissue (Kessler et al. 2018).
Flotillins in Cancer and as Regulators of MAP Kinase Signaling (R. Tikkanen)
Our findings show that depletion of flotillin-1 results in a severe impairment of EGF receptor signaling. Not only the activation of the EGF receptor is inhibited, but also the downstream signaling towards the MAP kinase cascade (Amaddii et al. 2012). We could show that flotillin-1 directly interacts with several proteins of the MAP kinase pathway, including CRAF, MEK1 and ERK2 and thus most likely functions in regulating the signaling at the level of MAPK signalosomes. Flotillins are frequently overexpressed in various types of cancers, and our research aims at characterizing the link between flotillin function and cancer/metastasis formation. In addition, flotillin-1 is involved in endosomal sorting of transmembrane proteins, such as the EGF receptor and interacts with endosomal sorting proteins such as the ESCRT complexes (Meister et al. 2017).
Our Funding
We gratefully acknowledge the support of following foundations and organizations:
German Research Council DFG Research Focus FOR 2497: http://gepris.dfg.de/gepris/projekt/289113135
Rare Trait Hope Fund: https://www.raretrait.com/home_1
Suomen AGU r.y.: http://www.aguyhdistys.com/p/etusivu.html
Jane ja Aatos Erkon Säätiö: https://jaes.fi/
SSADH-Defizit: https://ssadh.de/
SSADH Association: http://www.ssadh.net/site/c.ahJMLVMwGcK0E/b.8193477/k.B3DA/SSADH_Association.htm
In addition, we have several cooperation projects with the industry, as we are devoted to developing therapies for rare diseases!
Recent Publications (selection)
- Banning A, Tikkanen R (2021). Towards splicing therapy for lysosomal storage disorders: methylxanthines and luteolin ameliorate splicing defects in aspartylglucosaminuria and classic late infantile neuronal ceroid lipofuscinosis. Cells, 10, 2813; doi:103390/cells10112813
- Chen X, Snanoudj-Verber S, Pollard L, Hu Y, Cathey SS, Tikkanen R, Gray SJ. (2021) Preclinical Gene Therapy with AAV9/AGA in Aspartylglucosaminuria Mice Provides Evidence for Clinical Translation. Mol Ther, 29(3):989-1000. DOI: 10.1016/j.ymthe.2020.11.012
- Brennenstuhl H, Didiasova M, Assmann B, Bertoldi M, Molla G, Jung-Klawitter S, Hübschmann OK, Schröter J, Opladen T, Tikkanen R. (2020) Succinic Semialdehyde Dehydrogenase Deficiency: in vitro and in silico characterization of a novel pathogenic missense variant and analysis of the mutational spectrum of ALDH5A1. Int J Mol Sci, 21, 8578; doi:10.3390/ijms21228578
- Didiasova M, Banning A, Brennenstuhl H, Jung-Klawitter S, Cinquemani C, Opladen T, Tikkanen R. (2020)Succinic Semialdehyde Dehydrogenase Deficiency: an Update. (Review), Cells, 9(2). pii: E477. doi: 10.3390/cells9020477.
- Sairanen V, Tokola A, Tikkanen R, Laine M, Autti T. (2020) Statistical permutation test reveals progressive and region-specific iron accumulation in the thalami of children with aspartylglucosaminuria. Brain Sci, 10, 0677; doi:10.3390/brainsci10100677.
- Beckert B, Panico F, Pollmann R, Eming R, Banning A, Tikkanen R (2019) Immortalized human hTert/KER-CT keratinocytes as a model system for research on desmosomal adhesion and pathogenesis of Pemphigus Vulgaris, Int J Mol Sci, 20, 3113; doi:10.3390/ijms20133113
- Kessler EA, van Stuijvenberg L, van Bavel JJA, van Bennekom J, Zwartsen A, Rivaud MA, Vink A, Efimov IA, van Tintelen JP, Remme CA, Marc A. Vos MA, Banning A, de Boer TP, Tikkanen R, van Veen TAB. (2019)Flotillins in the intercalated disc are potential modulators of cardiac excitability. J Mol Cell Cardiol, 126:86-95. doi: 10.1016/j.yjmcc.2018.11.007
- Harjunen EL, Laine M, Tikkanen R, Helenius P (2019) Detailed Profile of Cognitive Dysfunction in Children with Aspartylglucosaminuria, J Inh Metab Dis, DOI: 10.1002/jimd.12159
- Tokola AM, Laine M, Tikkanen R, Autti TH (2019) Susceptibility-Weighted Imaging Findings in Aspartylglucosaminuria, Am J Neuroradiol, http://dx.doi.org/10.3174/ajnr.A6288
- Somogyi A, Petcherski A, Beckert B, Huebecker M, Priestman DA, Banning A, Cotman SL, Platt FM, Ruonala MO, Tikkanen R. (2018) Altered expression of ganglioside metabolizing enzymes results in GM3 ganglioside accumulation in cerebellar cells of a mouse model of juvenile neuronal ceroid lipofuscinosis. Int J Mol Sci, 19, 625; doi:10.3390/ijms19020625
- Banning A, Schiff M, Tikkanen R. (2018) Amlexanox Provides a Potential Therapy for Nonsense Mutations in the Lysosomal Storage Disorder Aspartylglucosaminuria. BBA - Molecular Basis of Disease, pii: S0925-4439(17)30463-5. doi: 10.1016/j.bbadis.2017.12.014
- Banning A, Babuke T, Kurrle N, Meister M, Ruonala MO, Tikkanen R. (2018) Flotillins regulate focal adhesions by interacting with α-actinin and by influencing the activation of Focal Adhesion Kinase. Cells, 7, 28; doi:10.3390/cells7040028
- Meister M, Baenfer S, Gärtner U, Koskimies J, Amaddii M, Jacob R, Tikkanen R. (2017) Regulation of cargo transfer between ESCRT-0 and ESCRT-I complexes by flotillin-1 during endosomal sorting of ubiquitinated cargo. Oncogenesis, 6, e344; doi:10.1038/oncsis.2017.47
- Banning A, König JF, Gray SJ, Tikkanen R. (2017) Functional Analysis of the Ser149/Thr149 Variants of Human Aspartylglucosaminidase and Optimization of the Coding Sequence for Protein Production. Int J Mol Sci, 18, 706; doi:10.3390/ijms18040706
- Banning A, Gülec C, Rouvinen J, Gray SJ, Tikkanen R. (2016) Identification of Small Molecule Compounds for Pharmacological Chaperone Therapy of Aspartylglucosaminuria. Sci Rep, 6, 37583; doi: 10.1038/srep37583
- Völlner F, Ali J, Kurrle N, Exner Y, Eming R, Hertl M, Banning A, Tikkanen R. (2016) Loss of flotillin expression results in weakened desmosomal adhesion and Pemphigus vulgaris-like localisation of desmoglein-3 in human keratinocytes. Sci Rep, 6, 28820; DOI:10.1038/srep28820
- Kapahnke M, Banning A, Tikkanen R. (2016) Random splicing of several exons caused by a single base change in the target exon of CRISPR/Cas9 mediated gene knockout. Cells 5, 45; doi:10.3390/cells5040045
- Banning A, Regenbrecht CRA, Tikkanen R. (2014) Increased activity of mitogen activated protein kinase pathway in flotillin-2 knockout mouse. Cell Signal, 26(2), 198-207. doi: 10.1016/j.cellsig.2013.11.001
- 18. Mooz J, Oberoi-Khanuja TK, Harms GS, Wang W, Tikkanen R, Jaiswal BS, Seshagiri S, Rajalingam K. (2014)Dimerization of ARAF promotes MAPK activation and cell migration. Science Signaling, 7(337):ra73. doi: 10.1126/scisignal.2005484
- Fork C, Hitzel J, Nichols BJ, Tikkanen R, Brandes RP. (2014) Flotillin-1 facilitates Toll-like receptor 3 signaling in human endothelial cells. Basic Res Cardiol, 109:439. doi: 10.1007/s00395-014-0439-4
For a complete list of publications of Ritva Tikkanen, see Pubmed.
Interested in joining us? Send an Email with your CV to Prof. Tikkanen!
Medical students: please inquire for vacancies for MD thesis!
Diploma/Master students are most welcome to join our dynamic crew!
PhD student candidates: please contact Prof. Tikkanen directly
