Reactive Intermediate and Prebiotic Chemistry
Singlet carbenes incorporating a divalent carbon atom (R–C–R’) have grown from laboratory curiosities and theoreticians’ pet peeves into reagents in the growing field of stable (i.e., heterocyclic) carbene chemistry. Still, the experimental characterization of many other simple yet fundamentally important reactive species such as alkyl- or hydroxycarbenes, silylenes or sulfenes is hampered by their high reactivity or lack of precursors: Hydroxycarbenes, for instance, have been an unknown class of compounds until 2008, when our group reported the synthesis and characterization of hydroxymethylene (H–C–OH), whose preparation has been challenging organic chemists for more than 80 years. The reaction of hydroxycarbene with formaldehyde would be a source of simple sugars (the so-called “formose reaction” in the origin of life theory). Considerable efforts are ongoing to understand the formation and distribution of a wide variety of simple organics in extraterrestrial environments, and the examination of the structures and reactivities of prototypes such as various hydroxycarbenes, simple carboxylic acids or seemingly "exotic" heteroatom-containing compounds may also provide glimpses of the prebiotic earth.
Reactions of the methylsulfinyl radical [CH3(O)S•] with oxygen (3O2) in solid argon
Hans Peter Reisenauer, Jarosław Romański, Grzegorz Mlostoń, Peter R. Schreiner
Chem. Commun. 2015, 51, 10022–10025. DOI: 10.1039/c5cc02168e
Highlight: Holm Petzold, Nachr. Chem. 2015, 63, 760
The atmospherically highly relevant methylsulfinyl radical (CH3(O)S•) readily reacts with molecular triplet oxygen in cryogenic argon matrices containing small amounts of 3O2. Comparison of experimental and computed IR- and UV/Vis spectra, including isotope exchange, show that the initially formed 3O2 adduct has the structure of a peroxyl radical (CH3(O)SOO•), which upon irradiation with UV light isom-erizes to the methylsulfonoxyl radical (CH3SO3•). The latter transforms into the methansulfonic acid radical (•CH2SO3H) by irradiation with visible light. During the matrix photolysis small amounts of SO3 and methyl radical were detected indi-cating competitive direct photodissociation.
Gas Phase Generation and Matrix Isolation of the Methylsulfonyl Radical CH3SO2• from Allylmethylsulfone
Hans-Peter Reisenauer, Jaroslaw Romanski, Peter R. Schreiner, and Grzegorz Mloston
J. Phys. Chem. A 2015, Article ASAP. DOI: 10.1021/jp5036647
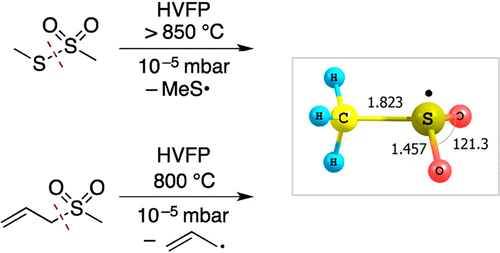
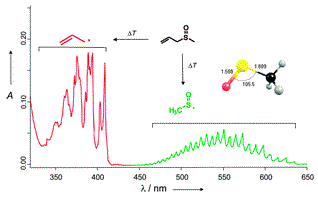
The atmospherically highly relevant methylsulfonyl radical (CH3SO2•) was generated by high-vacuum flash pyrolysis (HVFP) of allylmethylsulfone and isolated in an argon matrix at 10 K; the allyl radical formed as the cofragment. Upon thermolysis, the methylsulfonyl radical undergoes partial decomposition, leading to substantial amounts of sulfur dioxide in the matrix. The title compound was characterized through the assignment of eight fundamental IR bands of its CD3 and 13CH3 isotopologues and the excellent agreement with the B3LYP/6-311+G(3df,3pd) computed harmonic vibrational frequencies. The two most intense absorptions were found at 1267.1 and 1067.6 cm–1. In extension of this study S-methyl methanethiosulfonate was found to be another suitable, although less efficient, precursor for the gas-phase generation of the methylsulfonyl radical.
Gas phase preparation of carbonic acid and its monomethyl ester.
Hans Peter Reisenauer, Jan Philipp Wagner, Peter R. Schreiner
Angew. Chem. Int. Ed. 2014, 53, 11766–11771 (“hot paper”), DOI: 10.1002/anie.201406969.
Highlights:
a) Front cover of this issue;
b) Perspective: Götz Bucher and Wolfram Sander Science 2014, 346, 544–545; c) Feature: Carbonic Acid Crystal Forms Identified. Jyllian N. Kemsley C & EN News 2014, 92 (41), 28–29;
d) ChemistryViews: Carbonic Acid – And Yet It Exists!;
e) Innovations Report: Carbonic Acid—And Yet It Exists!;
f) Physorg.com: Preparation and characterization of gas-phase carbonic acid and its monomethyl ester.
 Carbonic acid (H2CO3), an essential molecule of life (e.g., as bicarbonate buffer), has been well characterized in solution and in the solid state, but for a long time, it has eluded its spectral characterization in the gas phase owing to a lack of convenient preparation methods; microwave spectra were recorded only recently. Here we present a novel and general method for the preparation of H2CO3 and its monomethyl ester (CH3OCO2H) through the gas-phase pyrolysis of di-tert-butyl and tert-butyl methyl carbonate, respectively. H2CO3 and CH3OCO2H were trapped in noble-gas matrices at 8 K, and their infrared spectra match those computed at high levels of theory [focal point analysis beyond CCSD(T)/cc-pVQZ] very well. Whereas the spectra also perfectly agree with those of the vapor phase above the β-polymorph of H2CO3, this is not true for the previously reported α-polymorph. Instead, the vapor phase above α-H2CO3 corresponds to CH3OCO2H, which sheds new light on the research that has been conducted on molecular H2CO3 over the last decades.
Carbonic acid (H2CO3), an essential molecule of life (e.g., as bicarbonate buffer), has been well characterized in solution and in the solid state, but for a long time, it has eluded its spectral characterization in the gas phase owing to a lack of convenient preparation methods; microwave spectra were recorded only recently. Here we present a novel and general method for the preparation of H2CO3 and its monomethyl ester (CH3OCO2H) through the gas-phase pyrolysis of di-tert-butyl and tert-butyl methyl carbonate, respectively. H2CO3 and CH3OCO2H were trapped in noble-gas matrices at 8 K, and their infrared spectra match those computed at high levels of theory [focal point analysis beyond CCSD(T)/cc-pVQZ] very well. Whereas the spectra also perfectly agree with those of the vapor phase above the β-polymorph of H2CO3, this is not true for the previously reported α-polymorph. Instead, the vapor phase above α-H2CO3 corresponds to CH3OCO2H, which sheds new light on the research that has been conducted on molecular H2CO3 over the last decades.
Combined ab initio molecular dynamics and experimental studies show that carbon atom addition to benzene.
Michael L. McKee, Hans-Peter Reisenauer, and Peter R. Schreiner,
J. Phys. Chem. A. 2014, 118, 2801–2809. DOI: 10.1021/jp501107b.
Car–Parrinello molecular dynamics was used to explore the reactions between triplet and singlet carbon atoms with benzene. The computations reveal that, in the singlet C atom reaction, products are very exothermic where nearly every collision yields a product that is determined by the initial encounter geometry. The singlet C atom reaction does not follow the minimum energy path because the bimolecular reaction is controlled by dynamics (i.e., initial orientation of encounter). On the other hand, in a 10 K solid Ar matrix, ground state C(3P) atoms do tend to follow RRKM kinetics. Thus, ab initio molecular dynamics (AIMD) results indicate that a significant fraction of C–H insertion occurs to form phenylcarbene whereas, in marked contrast to previous theoretical and experimental conclusions, the Ar matrix isolation studies indicate a large fraction of direct cycloheptatetraene formation, without the intermediacy of phenylcarbene. The AIMD calculations are more consistent with vaporized carbon atom experiments where labeling studies indicate the initial formation of phenylcarbene. This underlines that the availability of thermodynamic sinks can completely alter the observed reaction dynamics.
very exothermic where nearly every collision yields a product that is determined by the initial encounter geometry. The singlet C atom reaction does not follow the minimum energy path because the bimolecular reaction is controlled by dynamics (i.e., initial orientation of encounter). On the other hand, in a 10 K solid Ar matrix, ground state C(3P) atoms do tend to follow RRKM kinetics. Thus, ab initio molecular dynamics (AIMD) results indicate that a significant fraction of C–H insertion occurs to form phenylcarbene whereas, in marked contrast to previous theoretical and experimental conclusions, the Ar matrix isolation studies indicate a large fraction of direct cycloheptatetraene formation, without the intermediacy of phenylcarbene. The AIMD calculations are more consistent with vaporized carbon atom experiments where labeling studies indicate the initial formation of phenylcarbene. This underlines that the availability of thermodynamic sinks can completely alter the observed reaction dynamics.
Matrix Isolation and Spectroscopic Properties of the Methylsulfinyl Radical CH3(O)S•
Hans Peter Reisenauer,* Jaroslav Romanski, Grzegorz Mloston,* and Peter R. Schreiner,
Chem. Commun. 2013, 9467–9469. DOI: 10.1039/c3cc45379k
Thermolysis of 3,3,5,5-Tetramethyl-1,2,4-triothiolane-1-oxide: Matrix Isolation of the HOSS•-Radical.
Hans-Peter Reisenauer,* Grzegorz Mloston,* Jaroslaw Romanski, and Peter R. Schreiner,
Eur. J. Org. Chem. 2012, 3408–3415. DOI: 10.1002/ejoc.201200146
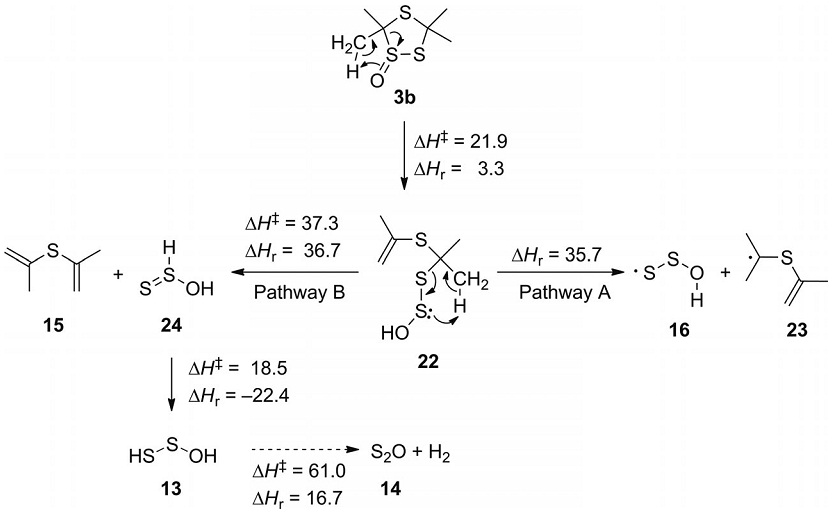
Flash vacuum pyrolysis of 3,3,5,5-tetramethyl-1,2,4-trithiolane 1-oxide performed at 700 °C yields the 1-oxatrisulfan-3-yl radical (HOSS·) along with disulfur monoxide (S2O) and diisopropyl sulfide, which were isolated in argon matrices at 10 K. Upon irradiation with UV light, the 1-oxatrisulfan-3-yl radical undergoes isomerization to the 1-oxatrisulfan-1-yl radical (HSSO·). Both radicals were identified by comparison of their computed and experimental IR and UV/Vis spectra. In addition, density functional theory (DFT) computations offer a plausible explanation of the most likely reaction mechanism, suggesting that the initial step is a 1,3-H shift with simultaneous ring opening. A 1-oxatrisulfane derivative formed thereby undergoes fragmentations via a radical and a competitive concerted pathway leading to the observed final products. The same mechanism also governs the thermal fragmentation of di-tert-butyl disulfide S-oxide. Its pyrolysis at 700 °C affords an analogous set of products, including the 1-oxatrisulfan-3-yl radical (HOSS·) as the key intermediate.
Conformations and Reactions of Bicyclo[3.2.1]oc-6-en-8-ylidene.
Udo H. Brinker, Alexander A. Bespokoev, Hans Peter Reisenauer, and Peter R. Schreiner
J. Org. Chem. 2012, 77, 3800–3807. DOI: 10.1021/jo3001035
![Conformations and Reactions of Bicyclo[3.2.1]oct-6-en-8-ylidene](https://www.uni-giessen.de/de/fbz/fb08/Inst/organische-chemie/agschreiner/research/images/carbenes/ylideneudo/@@images/image-0-71398ec3ce010e59ffd8ad9b31a86e11.jpeg) Bicyclo[3.2.1]oct-6-en-8-ylidene (1) can assume either the conformation of “classical” carbene 1a or that of foiled carbene 1b in which the divalent carbon bends toward the double bond. Oxadiazoline precursors for the generation of 1 were prepared, followed by photochemical and thermal decomposition as well as flash vacuum pyrolysis (FVP) of a tosyl hydrazone sodium salt precursor, to give a number of rearrangement products. Matrix isolation experiments demonstrate the presence of a diazo intermediate and methyl acetate in all photochemical and thermal precursor reactions. The major product from rearrangements of “classical” bridged carbene 1a is bicyclo[3.3.0]octa-1,3-diene as a result of an alkyl shift, while dihydrosemibullvalene formed from a 1,3-C–H insertion. In contrast, thus far unknown strained bicyclo[4.2.0]octa-1,7-diene formed by a vinyl shift in foiled carbene 1b. The experimental results are corroborated by density functional theory (DFT), MP2, and G4 computations.
Bicyclo[3.2.1]oct-6-en-8-ylidene (1) can assume either the conformation of “classical” carbene 1a or that of foiled carbene 1b in which the divalent carbon bends toward the double bond. Oxadiazoline precursors for the generation of 1 were prepared, followed by photochemical and thermal decomposition as well as flash vacuum pyrolysis (FVP) of a tosyl hydrazone sodium salt precursor, to give a number of rearrangement products. Matrix isolation experiments demonstrate the presence of a diazo intermediate and methyl acetate in all photochemical and thermal precursor reactions. The major product from rearrangements of “classical” bridged carbene 1a is bicyclo[3.3.0]octa-1,3-diene as a result of an alkyl shift, while dihydrosemibullvalene formed from a 1,3-C–H insertion. In contrast, thus far unknown strained bicyclo[4.2.0]octa-1,7-diene formed by a vinyl shift in foiled carbene 1b. The experimental results are corroborated by density functional theory (DFT), MP2, and G4 computations.
Oxathiirane
Peter R. Schreiner, Hans Peter Reisenauer, Jaroslaw Romanski, and Grzegorz Mloston
J. Am. Chem. Soc. 2010, 132, 7240–7241. Download
|
We describe the first preparation of the long sought after parent oxathiirane from sulfine through photochemical rearrangement with light at 313 ± 10 nm in an Ar-matrix at 11 K. Oxathiirane was characterized by the extraordinarily good agreement of experimentally measured and CCSD(T)/cc-pVTZ (unscaled) computed vibrational frequencies both for the perhydrogenated and perdeuterated species. The title molecule is about 10 kcal mol–1 less stable than sulfine, in marked contrast to the isomer energy difference of dioxirane vs. carbonyl oxide (ca. –25 kcal mol–1). This is due to the strong positive polarization (blue potential) vs. the highly electronegative oxygen atom (red). The stability ordering and the relative energy differences of carbonyl vs. thiocarbonyl groups underlines the likely role oxathiiranes play in sulfur transfer reactions. |
 |
Thermal Reactions of Regioisomeric 1,2,4-Trithiolane S-Oxides.
Grzegorz Mloston, Jaroslaw Romanski, Michael L. McKee, Hans-Peter Reisenauer, and Peter R. Schreiner
Eur. J. Org. Chem. 2010, 2132–2137. Download
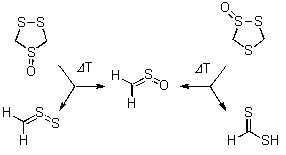
The products of the gas phase pyrolysis of two regioisomeric 1,2,4-trithiolane S-oxides were collected in an argon matrix at 10 K and studied by means of spectroscopic as well as computational methods. Whereas the main products of the pyrolysis of the ‘symmetrical’ S-oxide were identified as thioformaldehyde S-oxide and thioformaldehyde S-sulfide, the ‘non-symmetrical’ S-oxide gave predominantly dithioformic acid, which exists as a mixture of s-cis and s-trans conformers. We present a rationalization of the reaction pathways including density functional theory computations.
A formal carbon-sulfur triple bond: HCSOH.
Peter R. Schreiner, Hans Peter Reisenauer, Jaroslaw Romanski, and Grzegorz Mloston
Angew. Chem. Int. Ed. 2009, 48, 8133–8136. Download; Highlight: Neil Withers Nature Chem. 2009. Link; Robert Berger Nachr. Chem. 2010, 58, 7. Blog
|
Extremely rare: a CS triple bond can be assigned to HCSOH, a new molecule prepared through a photochemical [1,3]H-shift of sulfine H2C=S=O. But does this formal description agree with analyses on the basis of IR vibrations, bond lengths, bond orders, molecular orbitals, and compliance constants? Such types of molecules challenge and refine our current understanding of chemical bonding. |
 |
Infrared Signatures of the NCCO Radical.
|
|
Peter R. Schreiner, Hans Peter Reisenauer, Edit Mátyus, Attila G. Császár, Ali Siddiqi, Andrew C. Simmonett, and Wesley D. Allen Phys. Chem. Chem. Phys. 2009, 11, 10385-10390. Download |
|
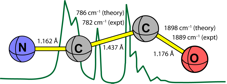
The first definitive infrared signatures of the elusive NCCO radical have been measured using a microwave discharge technique combined with low-temperature matrix-isolation spectroscopy, resulting in a consistent set of vibrational assignments for six isotopologues. The infrared spectra of these NCCO isotopologues were concomitantly established by rigorous variational nuclear-motion computations based on a high-level coupled-cluster quartic vibrational force field [ROCCSD(T)/cc-pCVQZ] and cubic dipole field [ROCCSD/cc-pCVTZ]. Our experimental and theoretical results for NCCO overturn the vibrational assignments in a NIST-JANAF compilation and those from a recent two-dimensional cross-spectral correlation analysis. For the parent isotopologue at 11 K in a nitrogen matrix, we find the signature bands n2(CO str.) = 1889.2 cm-1 and n3(CC str.) = 782.0 cm-1. Our variational vibrational computations reveal strong mixing of the n3 stretching fundamental and the n4+n5 bending combination level for all isotopologues. These Fermi resonances manifest a clear breakdown of the simple normal-mode picture of molecular vibrations at low energies. |
Prototypical Triplet Alkyl Phosphonatocarbenes.
Adelina Nemirowski, Hans Peter Reisenauer, Jaroslaw Romanski, Grzegorz Mloston, and Peter R. Schreiner,
J. Phys. Chem. A, 2008, 50, 13244. Download
|
Abstract. The current case study focuses on the generation, identification, and characterization of two representative mono- and disubstituted alkyl phosphonatocarbenes by means of matrix isolation techniques in conjunction with density functional theory [B3LYP/6-311++G(d,p)] and coupled cluster [CCSD(T)/cc-pVXZ, X = D, T] computations. The EPR measurements identify both carbenes as triplet ground-state species with D values of 0.660 and 0.623 cm–1 respectively, exhibiting persistency toward intramolecular reactions (the EPR signal observable in perfluoromethylcyclohexane up to around 70 K for the disubstituted molecule). While the reaction of the carbene center of the conformationally rich tetramethyl bisphosphonatocarbene with the CH bonds of the methyl groups leads to phosphaoxetane at room temperature, its fragmentation via a Wittig-type reaction during high vacuum flash pyrolysis (HVFP) results in dimethyl vinylphosphonate and methyl metaphosphate. The latter has been observed for the first time as an isolated entity. |
 |
Electronic Stabilization of Ground State Triplet Carbenes.
Adelina Nemirowski and Peter R. Schreiner, J. Org. Chem. 2007, 72, 9533. Download
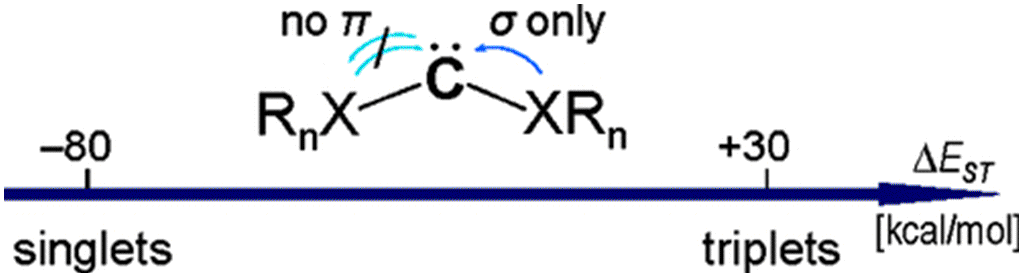
Abstract. Based on systematic ab initio (CCSD(T)/cc-pVDZ) studies of substituent effects, we present a concept for the construction of electronically stabilized triplet ground state carbenes with singlet−triplet energy separations (∆EST) exceeding that of methylene. Sterically demanding and conjugating substituents were excluded from the selection of model compounds under investigation, as these either destabilize both the singlet and the triplet states or delocalize unpaired spins away from the carbene carbon. Negative partial charges on the carbene center allow for the prediction of the electronic stabilization of substituted carbenes. To decrease carbene reactivity, we chose b-substituents with strong polar bonds. Among them, highly electronegative elements such as fluorine and oxygen enlarge the ∆EST value with respect to hydrogen, while chlorine does not due to p-orbital participation.
Dimethoxycarbene: Conformational Analysis of a Reactive Intermediate.
Hans Peter Reisenauer, Jaroslaw Romanski, Grzegorz Mloston, and Peter R. Schreiner,
Eur. J. Org. Chem. 2006, 4813. Download
Abstract. Dimethoxycarbene was prepared from an oxadiazoline precursor under high-vacuum flash pyrolysis (HVFP) conditions and was trapped at low temperatures by matrix isolation techniques (Ar, 10 K). The excellent agreement between the computed [CCSD(T)/cc-pVDZ] IR spectrum of the mixture of conformers of dimethoxycarbene and the experimentally measured IR absorptions allows a detailed analysis ofthe conformational preference of dimethoxycarbene. ItsUV spectrum is in agreement with earlier studies and our TD-B3LYP/6-311+G(d,p) computations. The computed [CCSD(T)/cc-pVDZ] rotational profile is rather steep and separates the s-trans,s-trans and s-cis,s-trans conformers by a 16 kcal mol-1 barrier, whilst the s-cis,s-cis conformer is too high-lying to be observable (+17 kcal mol-1). In marked contrast with the gauche,gauche minimum of dimethoxymethane, the s-trans,s-trans conformer of dimethoxycarbene is slightly preferred (0.5 kcal mol-1). The s-cis,s-trans conformer equilibrates at the high temperatures required during HFVP generation and both conformers can be identified in the IR spectrum of the argon matrix at 10 K. The conformational preference is partly due to the minimization of the overall dipole moment in the s-trans,s-trans conformer.
H–C–SiH3: Direct Generation and Spectroscopic Identification of Ethylidene’s Cousin.
Peter R. Schreiner, Hans Peter Reisenauer, Kurt W. Sattelmeyer, and Wesley D. Allen,
J. Am. Chem. Soc. 2005, 127, 12156. Download
|
Abstract. Ground-state triplet silaethylidene, generated directly by the reaction of 3P carbon atoms with silane under matrix isolation conditions in solid Ar (10–12 K), has been thoroughly characterized by the EPR and IR spectra of both the parent and perdeuterated isotopologs. A theoretical anharmonic vibrational analysis based on a CCSD(T)/cc-pVTZ complete quartic force field gave remarkable agreement with the experimental IR fundamentals, generally within 10 cm–1 and without any empirical scaling of the ab initio frequencies. Silaethylidene exhibits a CS minimum with a H–C–Si angle near 153 °, but the barrier to H–C–Si linearity (C3v symmetry) is only 0.24 kcal mol–1. This minuscule barrier can be surmounted by zero-point vibrations, as evident from the EPR data. The triplet stabilizing effect of the electropositive SiH3 group amounts to about 15 kcal mol–1. |
|
RI
Infrared Signatures of the NCCO Radical.
|
|
Peter R. Schreiner, Hans Peter Reisenauer, Edit Mátyus, Attila G. Császár, Ali Siddiqi, Andrew C. Simmonett, and Wesley D. Allen Phys. Chem. Chem. Phys. 2009, 11, 10385-10390. Download |
|
The first definitive infrared signatures of the elusive NCCO radical have been measured using a microwave discharge technique combined with low-temperature matrix-isolation spectroscopy, resulting in a consistent set of vibrational assignments for six isotopologues. The infrared spectra of these NCCO isotopologues were concomitantly established by rigorous variational nuclear-motion computations based on a high-level coupled-cluster quartic vibrational force field [ROCCSD(T)/cc-pCVQZ] and cubic dipole field [ROCCSD/cc-pCVTZ]. Our experimental and theoretical results for NCCO overturn the vibrational assignments in a NIST-JANAF compilation and those from a recent two-dimensional cross-spectral correlation analysis. For the parent isotopologue at 11 K in a nitrogen matrix, we find the signature bands n2(CO str.) = 1889.2 cm-1 and n3(CC str.) = 782.0 cm-1. Our variational vibrational computations reveal strong mixing of the n3 stretching fundamental and the n4+n5 bending combination level for all isotopologues. These Fermi resonances manifest a clear breakdown of the simple normal-mode picture of molecular vibrations at low energies. |
Prototypical Triplet Alkyl Phosphonatocarbenes.
Adelina Nemirowski, Hans Peter Reisenauer, Jaroslaw Romanski, Grzegorz Mloston, and Peter R. Schreiner,
J. Phys. Chem. A, 2008, 50, 13244. Download
|
Abstract. The current case study focuses on the generation, identification, and characterization of two representative mono- and disubstituted alkyl phosphonatocarbenes by means of matrix isolation techniques in conjunction with density functional theory [B3LYP/6-311++G(d,p)] and coupled cluster [CCSD(T)/cc-pVXZ, X = D, T] computations. The EPR measurements identify both carbenes as triplet ground-state species with D values of 0.660 and 0.623 cm–1 respectively, exhibiting persistency toward intramolecular reactions (the EPR signal observable in perfluoromethylcyclohexane up to around 70 K for the disubstituted molecule). While the reaction of the carbene center of the conformationally rich tetramethyl bisphosphonatocarbene with the CH bonds of the methyl groups leads to phosphaoxetane at room temperature, its fragmentation via a Wittig-type reaction during high vacuum flash pyrolysis (HVFP) results in dimethyl vinylphosphonate and methyl metaphosphate. The latter has been observed for the first time as an isolated entity. |
 |
Electronic Stabilization of Ground State Triplet Carbenes.
Adelina Nemirowski and Peter R. Schreiner, J. Org. Chem. 2007, 72, 9533. Download
Abstract. Based on systematic ab initio (CCSD(T)/cc-pVDZ) studies of substituent effects, we present a concept for the construction of electronically stabilized triplet ground state carbenes with singlet−triplet energy separations (∆EST) exceeding that of methylene. Sterically demanding and conjugating substituents were excluded from the selection of model compounds under investigation, as these either destabilize both the singlet and the triplet states or delocalize unpaired spins away from the carbene carbon. Negative partial charges on the carbene center allow for the prediction of the electronic stabilization of substituted carbenes. To decrease carbene reactivity, we chose b-substituents with strong polar bonds. Among them, highly electronegative elements such as fluorine and oxygen enlarge the ∆EST value with respect to hydrogen, while chlorine does not due to p-orbital participation.

Dimethoxycarbene: Conformational Analysis of a Reactive Intermediate.
Hans Peter Reisenauer, Jaroslaw Romanski, Grzegorz Mloston, and Peter R. Schreiner,
Eur. J. Org. Chem. 2006, 4813. Download
Abstract. Dimethoxycarbene was prepared from an oxadiazoline precursor under high-vacuum flash pyrolysis (HVFP) conditions and was trapped at low temperatures by matrix isolation techniques (Ar, 10 K). The excellent agreement between the computed [CCSD(T)/cc-pVDZ] IR spectrum of the mixture of conformers of dimethoxycarbene and the experimentally measured IR absorptions allows a detailed analysis ofthe conformational preference of dimethoxycarbene. ItsUV spectrum is in agreement with earlier studies and our TD-B3LYP/6-311+G(d,p) computations. The computed [CCSD(T)/cc-pVDZ] rotational profile is rather steep and separates the s-trans,s-trans and s-cis,s-trans conformers by a 16 kcal mol-1 barrier, whilst the s-cis,s-cis conformer is too high-lying to be observable (+17 kcal mol-1). In marked contrast with the gauche,gauche minimum of dimethoxymethane, the s-trans,s-trans conformer of dimethoxycarbene is slightly preferred (0.5 kcal mol-1). The s-cis,s-trans conformer equilibrates at the high temperatures required during HFVP generation and both conformers can be identified in the IR spectrum of the argon matrix at 10 K. The conformational preference is partly due to the minimization of the overall dipole moment in the s-trans,s-trans conformer.
H–C–SiH3: Direct Generation and Spectroscopic Identification of Ethylidene’s Cousin.
Peter R. Schreiner, Hans Peter Reisenauer, Kurt W. Sattelmeyer, and Wesley D. Allen,
J. Am. Chem. Soc. 2005, 127, 12156. Download
|
Abstract. Ground-state triplet silaethylidene, generated directly by the reaction of 3P carbon atoms with silane under matrix isolation conditions in solid Ar (10–12 K), has been thoroughly characterized by the EPR and IR spectra of both the parent and perdeuterated isotopologs. A theoretical anharmonic vibrational analysis based on a CCSD(T)/cc-pVTZ complete quartic force field gave remarkable agreement with the experimental IR fundamentals, generally within 10 cm–1 and without any empirical scaling of the ab initio frequencies. Silaethylidene exhibits a CS minimum with a H–C–Si angle near 153 °, but the barrier to H–C–Si linearity (C3v symmetry) is only 0.24 kcal mol–1. This minuscule barrier can be surmounted by zero-point vibrations, as evident from the EPR data. The triplet stabilizing effect of the electropositive SiH3 group amounts to about 15 kcal mol–1. |
|
RI
Singlet carbenes incorporating a divalent carbon atom (R–C–R’) have grown from laboratory curiosities and theoreticians’ pet peeves into reagents in the growing field of stable (i.e., heterocyclic) carbene chemistry. Still, the experimental characterization of many other simple yet fundamentally important reactive species such as alkyl- or hydroxycarbenes, silylenes or sulfenes is hampered by their high reactivity or lack of precursors: Hydroxycarbenes, for instance, have been an unknown class of compounds until 2008, when our group reported the synthesis and characterization of hydroxymethylene (H–C–OH), whose preparation has been challenging organic chemists for more than 80 years. The reaction of hydroxycarbene with formaldehyde would be a source of simple sugars (the so-called “formose reaction” in the origin of life theory). Considerable efforts are ongoing to understand the formation and distribution of a wide variety of simple organics in extraterrestrial environments, and the examination of the structures and reactivities of prototypes such as various hydroxycarbenes, simple carboxylic acids or seemingly "exotic" heteroatom-containing compounds may also provide glimpses of the prebiotic earth.
- Director: Univ.-Prof. Peter R. Schreiner
-
Co-workers:
Current work:
- Preparation and Characterization of the Enol of Acetamide: 1-Aminoethenol, a High-Energy Prebiotic Molecule
-
Artur Mardyukov, Felix Keul, and Peter R. Schreiner
Chem. Sci. 2020, xx, yy–zz. in press. DOI: 10.1039/D0SC04906AAmide tautomers, which constitute the higher-energy amide bond linkage, not only are key for a variety of biological but also prebiotic processes. In this work, we present the gas-phase synthesis of 1-aminoethenol, the higher-energy tautomer of acetamide, that has not been spectroscopically identified to date. The title compound was prepared by flash vacuum pyrolysis of malonamic acid and was characterized employing matrix isolation infrared as well as ultraviolet/visible spectroscopy. Coupled-cluster computations at the AE-CCSD(T)/cc-pVTZ level of theory support the spectroscopic assignments. Upon photolysis at λ > 270 nm, the enol rearranges to acetamide as well as ketene and ammonia. As the latter two are even higher in energy, they constitute viable starting materials for formation of the title compound.
- Interstellar Formation of Biorelevant Pyruvic Acid (CH3COCOOH)
-
Nils Fabian Kleimeier, André K. Eckhardt, Peter R. Schreiner and Ralf I. Kaiser
Chem 2020, xx,in press. DOI: 10.1016/j.chempr.2020.10.003One of the key questions is how life could have emerged on early Earth and what chemicals and key reactions were involved. Terrestrial biomolecules, such as DNA, RNA, and peptides, formed from building blocks like nucleobases and amino acids. But where do these come from? Simple chemical building blocks could have formed on icy grains in space and may have survived comet impact on the early Earth. Pyruvic acid is widely accepted as a key prebiotic starting material, as it may have served as a fundamental building block for biorelevant molecules. This is underlined by the identification of pyruvic acid in carbonaceous meteorites. This study investigates the formation of pyruvic acid under interstellar conditions to encourage scientists in other fields to consider pyruvic acid as a potential interstellar molecule and include it in their radio-astronomical line searches. For chemists, the study will lead to a better understanding of the fundamental processes of abiotic syntheses of organic molecules.
- Spectroscopic Identification of the •SSNO Isomers
-
Lina Wang, Zhuang Wu, Bo Lu, André K. Eckhardt, Peter R. Schreiner, Tarek Trabelsi, Joseph S. Francisco, Qian Yao, Hua Guo and Xiaoqing Zeng
J. Chem. Phys. 2020, 153, 094303. DOI: 10.1063/5.0020669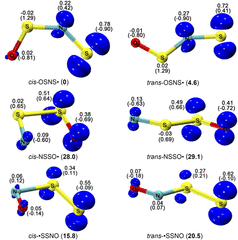 Elusive [S, S, N, O] isomers including the perthiyl radical •SSNO are S/N hybrid species in the complex bioinorganic chemistry of signaling molecules H2S and •NO. By mixing thermally generated disulfur (S2) with •NO in the gas phase, •SSNO was generated and subsequently isolated in cryogenic Ar- and N2-matrices at 10.0 K and 15.0 K, respectively. Upon irradiation with a 266 nm laser, •SSNO isomerizes to novel sulfinyl radicals cis-NSSO• and trans-NSSO• as well as thiyl radicals cis-OSNS• and trans-OSNS•, which have been characterized by combining matrix-isolation IR (15N-labeling) and UV/Vis spectroscopy and quantum chemical calculations at the CCSD(T)-F12/cc-pVTZ-F12 level of theory. The photo-induced reversible interconversion between NSSO• and OSNS• has also been observed.
Elusive [S, S, N, O] isomers including the perthiyl radical •SSNO are S/N hybrid species in the complex bioinorganic chemistry of signaling molecules H2S and •NO. By mixing thermally generated disulfur (S2) with •NO in the gas phase, •SSNO was generated and subsequently isolated in cryogenic Ar- and N2-matrices at 10.0 K and 15.0 K, respectively. Upon irradiation with a 266 nm laser, •SSNO isomerizes to novel sulfinyl radicals cis-NSSO• and trans-NSSO• as well as thiyl radicals cis-OSNS• and trans-OSNS•, which have been characterized by combining matrix-isolation IR (15N-labeling) and UV/Vis spectroscopy and quantum chemical calculations at the CCSD(T)-F12/cc-pVTZ-F12 level of theory. The photo-induced reversible interconversion between NSSO• and OSNS• has also been observed.
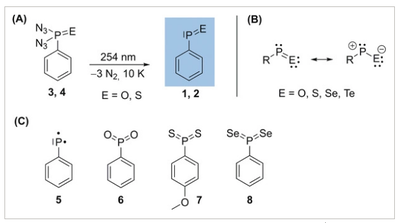
- Isolation and Characterization of the Free Phenylphosphinidene Chalcogenides C6H5P=O and C6H5P=S, the Phosphorous Analogues of Nitrosobenzene and Thionitrosobenzene
-
Artur Mardyukov, Felix Keul and Peter R. Schreiner
Angew. Chem. Int. Ed. 2020, 59, 12445–12449. DOI: 10.1002/anie.202004172The structures and reactivities of organic phosphinidene chalcogenides have been mainly inferred from trapping or complexation experiments. Phosphinidene chalcogenide derivatives appear to be an elusive family of molecules that have been suggested as reactive intermediates in multiple organophosphorus reactions. The quest to isolate “free” phosphinidene chalcogenides remains a challenge in the field. Here, we present the synthesis, IR, and UV/Vis spectroscopic identification of hitherto elusive phenylphosphinidene oxide and phenylphosphinidene sulfide from the corresponding phosphonic diazide precursors. We isolated these higher congeners of nitroso‐ and thionitrosobenzene in argon matrices at 10 K. The spectral assignments are supported by B3LYP/6–311++G(3df,3pd) and MP2/cc‐pVTZ computations.
- Photochemistry of HNSO2 in Cryogenic Matrices: Spectroscopic Identification of the Intermediates and Mechanism
-
Changyun Chen, Lina Wang, Xiaofang Zhao, Zhuang Wu, Bastian Bernhardt, André K. Eckhardt, Peter R. Schreiner, Xiaoqing Zeng
PCCP 2020, 22, 7975–7983. DOI: 10.1039/D0CP00962HSmall molecules solely consisting of H, N, O, and S are highly relevant intermediates in atmospheric chemistry and biology. Even though several isomers of [HNO2S] have been computationally predicted, only the IR spectra for the two lowest-energy isomers HNSO2 and syn–syn HONSO have been previously reported. Herein, the photochemistry (193 nm laser) of HNSO2 in N2-, Ne-, and Ar-matrices (≤15 K) has been studied. Aside from syn–syn HONSO, several new isomers including anti–syn HONSO, gauche–syn HOSNO, syn HOS(O)N, anti HOS(O)N, syn HS(O)NO, anti HN(O)SO, gauche–syn HSONO, and an elusive caged-radical pair HOS˙⋯˙NO have been identified. Additionally, the formation of fragments HONO, HO˙, ˙NO, and ˙NO2 has also been observed. The characterization of these species with matrix-isolation IR and UV/Vis spectroscopy is supported by 15N-labeling and quantum chemical computations at the B3LYP/6-311++G(3df,3pd) level. Furthermore, the photo-induced isomerization reactions, including the conformational conversion of syn–syn HONSO → anti–syn HONSO and reversible isomerization of HOSNO ↔ anti–syn HONSO, syn–syn HONSO ↔ HN(O)SO, HSONO ↔ HS(O)NO, and HOS˙⋯˙NO ↔ HOSNO have also been observed, and the underlying mechanism is discussed.
- The Elusive Cyclotriphosphazene Molecule and its Dewar-Benzene Type Valence Isomer (P3N3)
-
Cheng Zhu, André K. Eckhardt, Alexandre Bergantini, Santosh K. Singh, Peter R. Schreiner, Ralf I. Kaiser
Sci. Adv. 2020, 6, eaba6934. DOI: 10.1126/sciadv.aba6934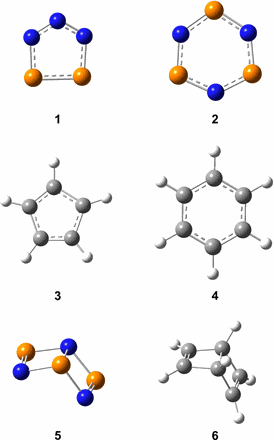
Although the chemistry of phosphorus and nitrogen has fascinated chemists for more than 350 years, the Hückel aromatic cyclotriphosphazene (P3N3, 2) molecule—a key molecular building block in phosphorus chemistry—has remained elusive. Here, we report a facile, versatile pathway producing cyclotriphosphazene and its Dewar benzene–type isomer (P3N3, 5) in ammonia-phosphine ices at 5 K exposed to ionizing radiation. Both isomers were detected in the gas phase upon sublimation via photoionization reflectron time-of-flight mass spectrometry and discriminated via isomer-selective photochemistry. Our findings provide a fundamental framework to explore the preparation of inorganic, isovalent species of benzene (C6H6) by formally replacing the C─H moieties alternatingly through phosphorus and nitrogen atoms, thus advancing our perception of the chemical bonding of phosphorus systems.
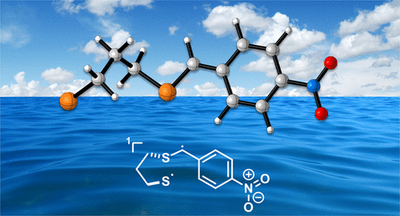
- Capture and reactivity of an elusive carbon-sulfur centered biradical
-
Dennis Gerbig, Bastian Bernhardt, Raffael C. Wende and Peter R. Schreiner
J. Phys. Chem. 2020, 124, 2014–2018. DOI: 10.1021/acs.jpca.9b11795The initial oxidation product of dimethyl sulfide in the marine boundary layer, the methyl thiomethyl radical, has remained elusive. A structurally analogous biradical with one radical center in the α-position to a sulfur atom could now be obtained by UV irradiation of p-nitrobenzaldehyde dithiane isolated in solid dinitrogen (N2) or Ar at cryogenic temperatures. A spin-forbidden reaction with triplet dioxygen (3O2) does not occur. The dithiane of o-nitrobenzaldehyde rather undergoes a series of rearrangements under the same conditions, resulting in overall photodeprotection.
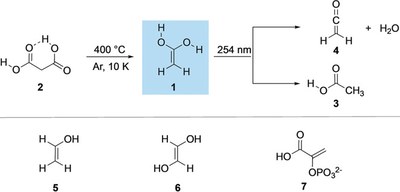
- 1,1-Ethenediol – The Long Elusive Enol of Acetic Acid
-
Artur Mardyukov, André K. Eckhardt, and Peter R. Schreiner
Angew. Chem. Int. Ed. 2020, 58, 5577–5580. DOI: 10.1002/anie.201915646We present the first spectroscopic identification of hitherto unknown 1,1‐ethenediol, the enol tautomer of acetic acid. The title compound was generated in the gas phase through flash vacuum pyrolysis of malonic acid at 400 °C. The pyrolysis products were subsequently trapped in argon matrices at 10 K and characterized spectroscopically by means of IR and UV/Vis spectroscopy together with matching its spectral data with computations at the CCSD(T)/cc‐pCVTZ and B3LYP/6–311++G(2d,2p) levels of theory. Upon photolysis at λ=254 nm, the enol rearranges to acetic acid and ketene.
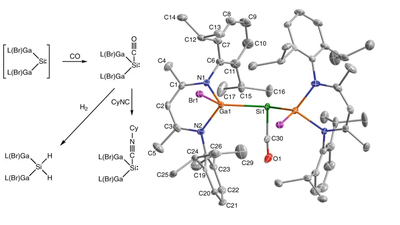
- A silicon–carbonyl complex stable at room temperature
-
Chelladurai Ganesamoorthy, Juliane Schoening, Christoph Wölper, Lijuan Song, Peter R. Schreiner, and Stephan Schulz
Nat. Chem. 2020, 12, 608–614. DOI: 10.1038/s41557-020-0456-x
Highlights: a) Leigh Krietsch Boerner Chem. Eng. News. 2020, 98 (16), 9; b) David Schilter Nat. Rev. Chem. 2020, 4, 274; c) Nachr. Chem. 2020, 68, 42.Main-group-element compounds with energetically high-lying donor and low-lying acceptor orbitals are able to mimic chemical bonding motifs and reactivity patterns known in transition metal chemistry, including small-molecule activation and catalytic reactions. Monovalent group 13 compounds and divalent group 14 compounds, particularly silylenes, have been shown to be excellent candidates for this purpose. However, one of the most common reactions of transition metal complexes, the direct reaction with carbon monoxide and formation of room-temperature isolable carbonyl complexes, is virtually unknown in main-group-element chemistry. Here, we show the synthesis, single-crystal X-ray structure, and density functional theory computations of a room-temperature-stable silylene carbonyl complex [L(Br)Ga]2Si:–CO (L = HC[C(Me)N(2,6-iPr2-C6H3)]2), which was obtained by direct carbonylation of the electron-rich silylene intermediate [L(Br)Ga]2Si:. Furthermore, [L(Br)Ga]2Si:–CO reacts with H2 and PBr3 with bond activation, whereas the reaction with cyclohexyl isocyanide proceeds with CO substitution.
- Generation and Spectroscopic Identification of the Thiuram Radical (CH3)2NCS2∙
-
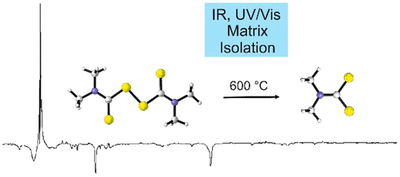
Artur Mardyukov, Felix Keul, and Peter R. Schreiner
J. Phys. Chem. A 2019, 123, 4937–4941. DOI: 10.1021/acs.jpca.9b03307We report the first preparation, matrix-isolation, and IR and UV/vis spectroscopic characterization of the thiuram radical that is a highly important species for many industrial processes. The thiuram radical was prepared by thermal dissociation of tetramethylthiuram disulfide and was identified by matching its spectroscopic data with density functional theory [UB3LYP/6-311++G(3df,3pd)] computations. The title compound proved to be highly photolabile, and irradiation with light at λ = 623 nm affords a hitherto unknown carbamodithioic acid, N-(methyl)-N-methyl radical, as characterized by IR and UV/vis spectroscopy in low-temperature matrices.
- Spectroscopic Identification of the Phenyltelluryl Radical and its Reactivity Toward Molecular Oxygen
-
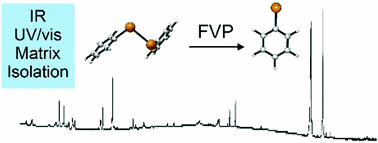
Felix Keul, Artur Mardyukov and Peter R. Schreiner
PCCP 2019, 21, 25797–25801. DOI: 10.1039/C9CP05112KThe phenyltelluryl radical was prepared by high-vacuum flash pyrolysis of diphenyl ditelluride and was chacracterized by matrix isolation IR and UV/Vis spectroscopy. After doping the matrix with molecular oxygen and allowing bimolecular reactions, the hitherto unkown phenyltelluro peroxy radical formed and was identified via IR spectroscopy. Irradiation with light at λ = 436 nm leads to isomerization to the thermodynamically more stable novel phenyltelluroyl radical. All experimental findings agree well with density functional theory (UB3LYP/Def2QZVPP and UM06-2X/Def2QZVPP) computations.
- Formation of Glyoxylic Acid (HCOCOOH) in Interstellar Ices – A Key Entry Point for Prebiotic Chemistry
-
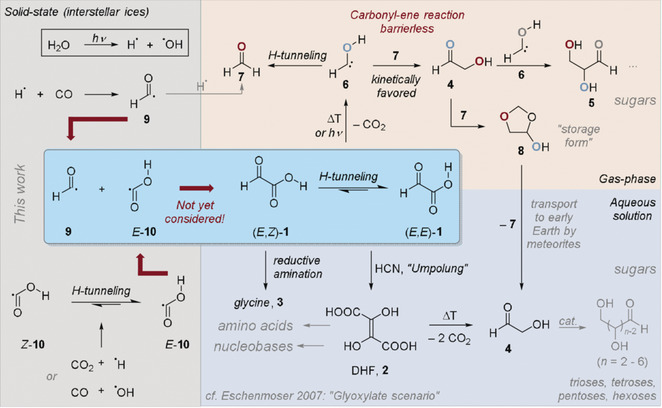
André K. Eckhardt, Alexandre Bergantini, Santosh K. Sing, Peter R. Schreiner and Ralf I. Kaiser
Angew. Chem. Int. Ed. 2019, 58, 5663–5667. DOI: 10.1002/anie.201901059With nearly 200 molecules detected in interstellar and circumstellar environments, the identification of the biologically relevant α‐keto carboxylic acid, glyoxylic acid (HCOCOOH), is still elusive. Herein, the formation of glyoxylic acid via cosmic‐ray driven, non‐equilibrium chemistry in polar interstellar ices of carbon monoxide (CO) and water (H2O) at 5 K via barrierless recombination of formyl (HCO) and hydroxycarbonyl radicals (HOCO) is reported. In temperature‐programmed desorption experiments, the subliming neutral molecules were selectively photoionized and identified based on the ionization energy and distinct mass‐to‐charge ratios in combination with isotopically labeled experiments exploiting reflectron time‐of‐flight mass spectrometry. These studies unravel a key reaction path to glyoxylic acid, an organic molecule formed in interstellar ices before subliming in star‐forming regions like SgrB2(N), thus providing a critical entry point to prebiotic organic synthesis.
- Caged Nitric Oxide-Thiyl Radical Pairs
-
Zhuang Wu, Changyun Chen, Jie Liu, Yan Lu, Jian Xu, Jian, Xiangya Liu, Ganglong Cui, Tarek Trabelsi, Joseph Francisco, Artur Mardyukov, André K. Eckhardt, Peter R. Schreiner, Xiaoqing Zeng
J. Am. Chem. Soc. 2019, 141, 3361–3365. DOI: 10.1021/jacs.8b12746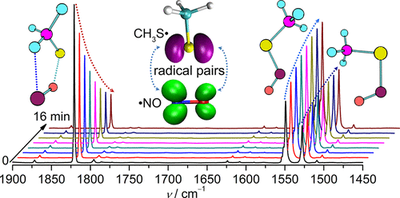
S-Nitrosothiols (RSNO) are exogenous and endogenous sources of nitric oxide in biological systems due to facile homolytic cleavage of the S–N bonds. By following the photolytic decomposition of prototypical RSNO (R = Me and Et) in Ne, Ar, and N2 matrixes (<10 K), elusive caged radical pairs consisting of nitric oxide (NO•) and thiyl radicals (RS•), bridged by O···S and H···N connections, were identified with IR and UV/vis spectroscopy. Upon red-light irradiation, both caged radical pairs (RS•···•ON) vanish and reform RSNO. According to the calculation at the CASPT2(10,8)/cc-pVDZ level (298.15 K), the dissociation energy of MeS•···•ON amounts to 4.7 kcal mol–1.
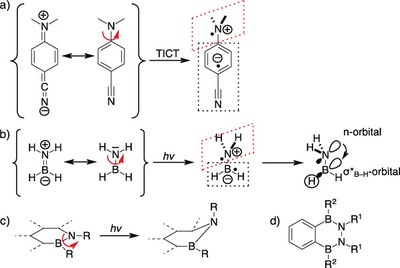
- Control of excited state conformations of B,N-heterocycles
-
Zhenpin Lu, Julia Ruhl, Henrik Quanz, Georg Albrecht, Christian Logemann, Derck Schlettwein, Peter R. Schreiner and Hermann A. Wegner
Angew. Chem. Int. Ed. 2019, 58, 4259–4263. DOI: 10.1002/anie.201814104We present a new concept to control the conformations of molecules in the excited state through harvesting negative hyperconjugation. The strategy was realized with the 2,3,1,4‐benzodiazadiborinane scaffold, which was prepared by a new synthetic procedure. Photochemical studies identified dual light emission, which was assigned to well‐defined conformers. The emission at longer wavelength can be switched off by restricting the rotational degrees of freedom in the solid state as well as by controlling the energy levels of the excited states through adjusting the solvent polarity.
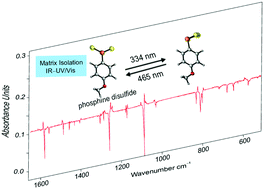
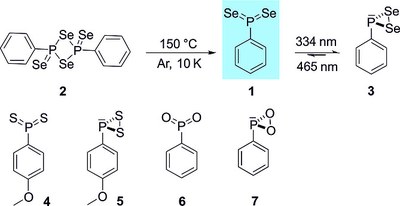
- Preparation and Characterization of Phenyl Phosphine Diselenide – The Monomeric Form of Woollins' Reagent
-
Artur Mardyukov, Felix Keul and Peter R. Schreiner
Eur. J. Org. Chem. 2019, 46, 387-390. DOI: 10.1002/ejoc.201800639We report the preparation, matrix‐isolation, and IR and UV/Vis spectroscopic characterization of phenyl phosphine diselenide, thus providing the first experimental evidence of the monomeric form of Woollins' reagent. Phenyl phosphine diselenide was prepared by thermal dissociation of Woollins' reagent and was identified by matching its spectroscopic data with density functional theory [B3LYP‐D3/6‐311++G(3df,3pd)] computations. The title compound proved to be highly photolabile and irradiation with light at λ = 334 nm results in the formation of hitherto unknown phenyldiselenyl phosphirane. Upon λ = 465 nm irradiation it rearranges back to phenyl phosphine diselenide.
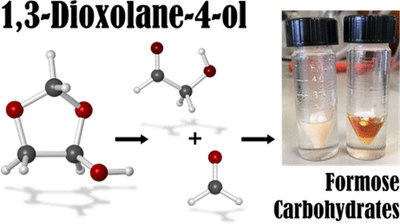
- 1,3-Dioxolane-4-ol Hemiacetal Stores Formaldehyde and Glycolaldhyde in the Gas Phase
-
André K. Eckhardt, Raffael C. Wende and Peter R. Schreiner
J. Am. Chem. Soc. 2018, 140,12333–12336. DOI: 10.1021/jacs.8b07480. Highlight: Cover picture of this issue.We report the spontaneous gas-phase formation of 1,3-dioxolane-4-ol, a mixed hemiacetal resulting from the addition of glycolaldehyde to formaldehyde. It was spectroscopically characterized by matching matrix IR spectra with coupled cluster computations. The formation of the hemiacetal must be surface-catalyzed owing to the very high computed reaction barrier of 39.8 kcal mol–1. The reaction barrier is lowered by almost 20 kcal mol–1 when a single water molecule acts as a proton shuttle in a favorable six-membered transition state. We characterized the hemiacetal in solution via NMR spectroscopy and followed its decomposition into its constituents within a few hours; it also dissociates upon contact with water. Sugars form in the presence of Ca(OH)2, in line with formose-type reactivity. 1,3-Dioxolane-4-ol may be considered a storage form for formaldehyde and glycolaldehyde that is rather stable in the gas-phase.
- Phenylsulfinyl Radical: Gas-Phase Generation, Photoisomerization and Oxidation
-
Jian Xu, Zhuang Wu, Huabin Wan, Guohai Deng, Bo Lu, André K. Eckhardt, Peter R. Schreiner, Tarek Trabelsi, Joseph S. Francisco and Xiaoqing Zeng
J. Am. Chem. Soc. 2018, 140, 9972–9978. DOI: 10.1021/jacs.8b05055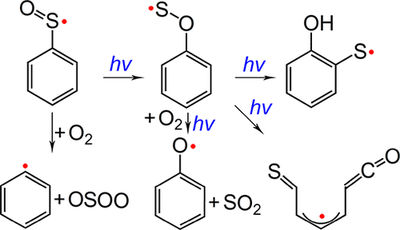
Arylsulfinyl radicals are key intermediates in sulfoxide chemistry. The parent molecule, phenylsulfinyl radical PhSO•, has been generated for the first time in the gas phase through high-vacuum flash pyrolysis of PhS(O)R (R = CF3 and Cl) at about 1000 K. Upon UV light irradiation (365 nm), PhSO• isomerizes to novel oxathiyl radical PhOS• in cryogenic matrices (2.8 K). Prolonged irradiation causes further isomerization of PhOS• to 2-hydroxyphenylthiyl radical, the formation of which has been also observed in the 193 nm laser photolysis of matrix-isolated 2-hydroxybenzenethiol. Concomitantly, ring-opening occurs during the UV photolysis of PhOS• and 2-hydroxybenzenethiol and forms an acyclic thioketoketene radical. Phenylsulfinyl radical reacts partially with molecular oxygen in the gas phase and yields phenyl radical Ph• and OSOO. Upon irradiation (365 nm), the isomeric oxathiyl radical also combines O2 with immediate dissociation to phenoxy radical PhO• and SO2. The identification of the intermediates with IR and UV–vis spectroscopy is supported by quantum chemical computations at the B3LYP/def2-TZVPP and UCCSD(T)/aug-cc-pV(D+d)Z levels of theory. The isomerization of PhSO• has been discussed based on the computed potential energy profile and the comparison with the intensively explored photochemistry of phenylperoxy radical PhOO•.
- Making Glycine Methyl Ester Chiral
-
Dennis Gerbig, Sarina Desch and Peter R. Schreiner
Chem. Eur. J. 2018, 24, 11904-11907. DOI: 10.1002/chem.201802119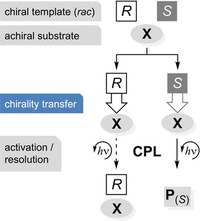
We demonstrate that the simple achiral amino acid glycine as its methyl ester inherits the chiral imprint of methyl lactate upon complexation, resulting in induced vibrational optical activity of the methylene C−H bonds. To mimic conditions of ice on comets that are considered long‐term reaction as well as storage entities for (organic) molecules, we employ the matrix isolation technique in conjunction with vibrational circular dichroism spectroscopy and DFT computations. The observed chirality transfer is likely a key element for the realization of concepts rationalizing chirogenesis, that is, the generation of a chiral imbalance.
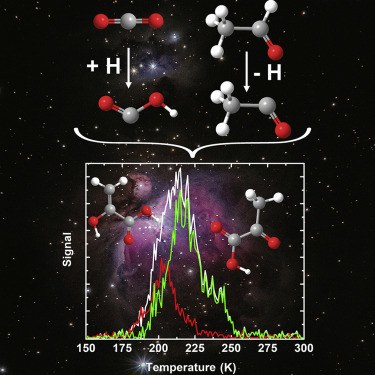
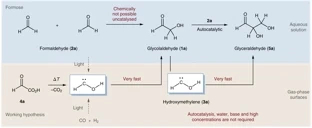
- Gas-phase sugar formation using hydroxymethylene as the reactive formaldehyde isomer.
-
André K. Eckhardt, Michael M. Linden, Raffael C. Wende, Bastian Bernhardt and Peter R. Schreiner
Nature Chem. 2018, 10, 1141–1147. DOI: 10.1038/s41557-018-0128-2Carbohydrates (CH2O)n are the formal adducts of carbon (atoms) to water with a repeating unit that structurally resembles H–C̈–OH (hydroxymethylene). Although hydroxymethylene has been suggested as a building block for sugar formation, it is a reactive species that had escaped detection until recently. Here we demonstrate that formaldehyde reacts with its isomer hydroxymethylene to give glycolaldehyde in a nearly barrierless reaction. This carbonyl–ene-type transformation operates in the absence of base and solvent at cryogenic temperatures similar to those found in extraterrestrial environments or interstellar clouds. Hydroxymethylene acts as a building block for an iterative sugar synthesis, as we demonstrate through the formation of the triose glyceraldehyde. The thermodynamically preferred ketose dihydroxyacetone does not form, and the formation of further branched sugars in the iterative synthesis presented here is unlikely. The results therefore provide a link between the well-known formose (Butlerow) reaction and sugar formation under non-aqueous conditions.
- The Near-UV Absorber OSSO and its Isomers
-
Zhuang Wu, Huabin Wan, Jian Xu, Bo Lu, André K. Eckhardt, Peter R. Schreiner, Changjian Xie, Hua Guo and Xiaoqing Zeng
Chem. Commun. 2018, 54, 4517–4520. DOI: 10.1039/C8CC00999FHighlight: Venusian near-UV absorber mystery solved in Chemistry World 2018, March 09, 2018.
Disulfur dioxide, OSSO, has been proposed as the enigmatic “near-UV absorber” in the yellowish atmosphere of Venus. However, the fundamentally important spectroscopic properties and photochemistry of OSSO are scarcely documented. By either condensing gaseous SO or 266 laser photolysis of an S2⋯O2 complex in Ar or N2 at 15 K, syn-OSSO, anti-OSSO, and cyclic OS(
![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O)S were identified by IR and UV/Vis spectroscopy for the first time. The observed absorptions (λmax) for OSSO at 517 and 390 nm coincide with the near-UV absorption (320–400 nm) found in the Venus clouds by photometric measurements with the Pioneer Venus orbiter. Subsequent UV light irradiation (365 nm) depletes syn-OSSO and anti-OSSO and yields a fourth isomer, syn-OSOS, with concomitant dissociation into SO2 and elemental sulfur.
O)S were identified by IR and UV/Vis spectroscopy for the first time. The observed absorptions (λmax) for OSSO at 517 and 390 nm coincide with the near-UV absorption (320–400 nm) found in the Venus clouds by photometric measurements with the Pioneer Venus orbiter. Subsequent UV light irradiation (365 nm) depletes syn-OSSO and anti-OSSO and yields a fourth isomer, syn-OSOS, with concomitant dissociation into SO2 and elemental sulfur.
- Unravelling Lawesson’s Reagent – The Structure of Monomeric (4-Methoxyphenyl)phosphine Disulfide
-
Artur Mardyukov, Dominik Niedek and Peter R. Schreiner
Chem. Commun. 2018, 54, 2715–2718. DOI: 10.1039/c8cc00034d
Highlights: a) Front cover of corresponding issue; b) “HotChem” article featured by the Royal Society of Chemistry.We describe the isolation as well as IR and UV/Vis spectroscopic characterization of (4-methoxyphenyl)phosphine disulfide in argon matrices at 10 K. The title compound proved to be highly photolabile; irradiation with UV light (λ = 334 nm) led to rearrangement to the equally unreported 3-(4-methoxyphenyl)-1,2,3-dithiaphosphirane. Photoreversion can be achieved upon irradiation at λ = 465 nm.
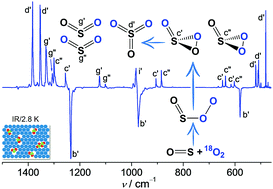
- Capture of SO3 Isomers in the Oxidation of Sulfur Monoxide with Molecular Oxygen
-
Zhuang Wu, Bo Lu, Ruijuan Feng, Jian Xu, Yan Lu, Huabin Wan, André K. Eckhardt, Peter R. Schreiner, Changjian Xie, Hua Guo, and Xiaoqing Zeng
Chem. Commun. 2018, 54, 1690–1693. DOI: 10.1039/C7CC09389F
Highlight: Inside front cover.When mixing SO with O2 in N2, Ne, or Ar, an end-on complex OS–OO forms in the gas phase and can subsequently be trapped at cryogenic temperatures (2.8–15.0 K). Upon infrared light irradiation, OS–OO converts to SO3 and SO2 + O with the concomitant formation of a rare 1,2,3-dioxathiirane 2-oxide, i.e., cyclic OS(
![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O)O. Unexpectedly, the ring-closure of 16OS–18O18O yields a ca. 2 : 1 mixture of cyclic 18OS(
O)O. Unexpectedly, the ring-closure of 16OS–18O18O yields a ca. 2 : 1 mixture of cyclic 18OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) 16O)18O and 16OS(
16O)18O and 16OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) 18O)18O. The characterization of OS–OO and OS(
18O)18O. The characterization of OS–OO and OS(![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O)O with IR and UV/Vis spectroscopy is supported by high-level ab initio computations.
O)O with IR and UV/Vis spectroscopy is supported by high-level ab initio computations.
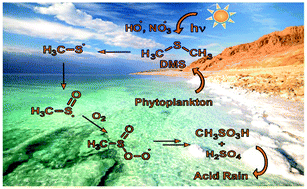
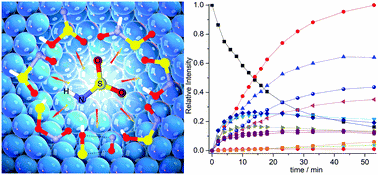
- Atmospherically Relevant Radicals Derived from the Oxidation of Dimethyl Sulfide
-
Artur Mardyukov and Peter R. Schreiner
Acc. Chem. Res. 2018, 51, 475–483. DOI: 10.1021/acs.accounts.7b00536The large number and amounts of volatile organosulfur compounds emitted to the atmosphere and the enormous variety of their reactions in various oxidation states make experimental measurements of even a small fraction of them a daunting task. Dimethyl sulfide (DMS) is a product of biological processes involving marine phytoplankton, and it is estimated to account for approximately 60% of the total natural sulfur gases released to the atmosphere. Ocean-emitted DMS has been suggested to play a role in atmospheric aerosol formation and thereby cloud formation. The reaction of ·OH with DMS is known to proceed by two independent channels: abstraction and addition. The oxidation of DMS is believed to be initiated by the reaction with
 ·OH and NO3· radicals, which eventually leads to the formation of sulfuric acid (H2SO4) and methanesulfonic acid (CH3SO3H). The reaction of DMS with NO3· appears to proceed exclusively by hydrogen abstraction. The oxidation of DMS consists of a complex sequence of reactions. Depending on the time of the day or altitude, it may take a variety of pathways. In general, however, the oxidation proceeds via chains of radical reactions. Dimethyl sulfoxide (DMSO) has been reported to be a major product of the addition channel. Dimethyl sulfone (DMSO2), SO2, CH3SO3H, and methanesulfinic acid (CH3S(O)OH) have been observed as products of further oxidation of DMSO. Understanding the details of DMS oxidation requires in-depth knowledge of the elementary steps of this seemingly simple transformation, which in turn requires a combination of experimental and theoretical methods. The methylthiyl (CH3S·), methylsulfinyl (CH3SO·), methylsulfonyl (CH3SO2·), and methylsulfonyloxyl (CH3SO3·) radicals have been postulated as intermediates in the oxidation of DMS. Therefore, studying the chemistry of sulfur-containing free radicals in the laboratory also is the basis for understanding the mechanism of DMS oxidation in the atmosphere. The application of matrix-isolation techniques in combination with quantum-mechanical calculations on the generation and structural elucidation of CH3SOx (x = 0–3) radicals is reviewed in the present Account. Experimental matrix IR and UV/vis data for all known species of this substance class are summarized together with data obtained using other spectroscopic techniques, including time-resolved spectroscopy, electron paramagnetic resonance spectroscopy, and others. We also discuss the reactivity and experimental characterization of these species to illustrate their practical relevance and highlight spectroscopic techniques available for the elucidation of their geometric and electronic structures. The present Account summarizes recent results regarding the preparation, characterization, and reactivity of various radical species with the formula CH3SOx (x = 0–3).
·OH and NO3· radicals, which eventually leads to the formation of sulfuric acid (H2SO4) and methanesulfonic acid (CH3SO3H). The reaction of DMS with NO3· appears to proceed exclusively by hydrogen abstraction. The oxidation of DMS consists of a complex sequence of reactions. Depending on the time of the day or altitude, it may take a variety of pathways. In general, however, the oxidation proceeds via chains of radical reactions. Dimethyl sulfoxide (DMSO) has been reported to be a major product of the addition channel. Dimethyl sulfone (DMSO2), SO2, CH3SO3H, and methanesulfinic acid (CH3S(O)OH) have been observed as products of further oxidation of DMSO. Understanding the details of DMS oxidation requires in-depth knowledge of the elementary steps of this seemingly simple transformation, which in turn requires a combination of experimental and theoretical methods. The methylthiyl (CH3S·), methylsulfinyl (CH3SO·), methylsulfonyl (CH3SO2·), and methylsulfonyloxyl (CH3SO3·) radicals have been postulated as intermediates in the oxidation of DMS. Therefore, studying the chemistry of sulfur-containing free radicals in the laboratory also is the basis for understanding the mechanism of DMS oxidation in the atmosphere. The application of matrix-isolation techniques in combination with quantum-mechanical calculations on the generation and structural elucidation of CH3SOx (x = 0–3) radicals is reviewed in the present Account. Experimental matrix IR and UV/vis data for all known species of this substance class are summarized together with data obtained using other spectroscopic techniques, including time-resolved spectroscopy, electron paramagnetic resonance spectroscopy, and others. We also discuss the reactivity and experimental characterization of these species to illustrate their practical relevance and highlight spectroscopic techniques available for the elucidation of their geometric and electronic structures. The present Account summarizes recent results regarding the preparation, characterization, and reactivity of various radical species with the formula CH3SOx (x = 0–3).
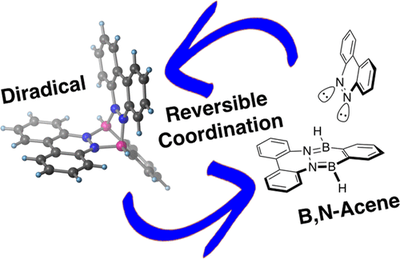
- A Stable Organic Neutral Diboron Diradical via Reversible Coordination
-
Zhenpin Lu, Henrik Quanz, Olaf Burghaus, Jonas Hoffmann, Christian Logemann, Peter R. Schreiner, Sebastian Beeck and Hermann A. Wegner
J. Am. Chem. Soc. 2018, 139, 18488–18491. DOI: 10.1021/jacs.7b11823We report the formation of a stable neutral diboron diradical simply by coordination of an aromatic dinitrogen compound to an ortho-phenyldiborane. This process is reversible upon addition of pyridine. The diradical species is stable above 200 °C. Computations are consistent with an open-shell triplet diradical with a very small open-shell singlet–triplet energy gap that is indicative of the electronic disjointness of the two radical sites. This opens a new way of generating stable radicals with fascinating electronic properties useful for a large variety of applications.
- The Trifluoromethyl Sulfinyl and Oxythiyl Radicals
-
Zhuang Wu, Jian Xu, Guohai Deng, Yan Lu, Liubov Sokolenko, Tarek Trabelsi, Joseph S. Francisco, André Kristopher Eckhardt, Peter R. Schreiner and Xiaoqing Zeng
Chem. Eur. J. 2018, 24, 1505-1508. DOI: 10.1002/chem.201705142Highlight: Cover feature DOI: 10.1002/chem.201705533

The trifluoromethyl sulfinyl radical and the trifluoromethyl oxathiyl radical, were generated in the gas phase and then trapped in solid noble gas matrices for characterization with IR and UV/Vis spectroscopy. Subsequent UV light irradiation resulted in an unprecedented isomerization from a sulfinyl radical to an oxathiyl radical by surmounting a formidable activation barrier, despite that such geometrical transformation has been computationally predicted for decades.
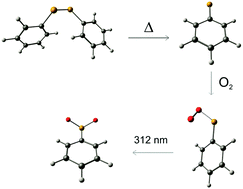
- The Phenylselenyl Radical and Its Reaction with Molecular Oxygen
-
Artur Mardyukov,Yetsedaw Tsegaw, Wolfram Sander and Peter R. Schreiner
PCCP 2017, 19, 27384-27388. DOI: 10.1039/C7CP05546CThe current study focuses on the generation, identification, and characterization of the phenylselenyl radical using the matrix isolation technique in combination with density functional theory (B3LYP/cc-pVTZ) computations. The hitherto unknown phenylselenyl peroxy radical was synthesized by co-condensation of the phenylselenyl radical with molecular ground state triplet oxygen from the gas phase and subsequent trapping in argon matrices at 10 K. The experimental IR spectra including 18O isotopically labelled materials compare well with the data obtained from B3LYP/cc-pVTZ computations. Upon 312 nm irradiation, the phenylselenyl peroxy radical isomerizes to the thermodynamically more stable equally novel phenylselenoyl radical.
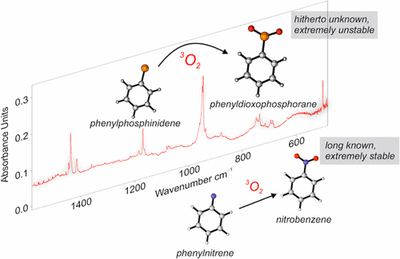
- Preparation and Characterization of Parent Phenylphosphinidene and its Oxidation to Phenyldioxophosphorane, the Elusive Phosphorous Analogue of Nitrobenzene
-
Artur Mardyukov, Dominik Niedek and Peter R. Schreiner
J. Am. Chem. Soc. 2017, 139,5019–5022. DOI: 10.1021/jacs.7b01639Triplet phenylphosphinidene was prepared by light-induced elimination of ethylene from the corresponding phenylphosphirane and was characterized by IR and UV/vis spectroscopy together with matching of its spectral data with density functional theory computations. The photolysis of phenylphosphirane in 3P-O2 doped matrices enabled the spectroscopic identification of a hitherto unknown phenyldioxophosphorane, the long elusive phosphorus analogue of nitrobenzene.
- Towards the Pyrolytic Preparation of Carbonothioic O,O-Acid (Monothiocarbonic Acid)
-
Dominik Niedek, J. Philipp Wagner, and Peter R. Schreiner*
J. Anal. Appl. Pyrol. 2017, 124, 439–445. DOI: 10.1016/j.jaap.2017.02.024Carbonothioic O,O-acid (monothiocarbonic acid), the sulfur analogue of carbonic acid, is thus far an experimentally unreported molecule of atmospheric and biochemical relevance. Computations at the CCSD(T)/cc-pVQZ//CCSD(T)/cc-pVTZ level of theory reveal that its carbonothioic O,S-acid tautomer is energetically favored over the carbonothioic O,O-acid tautomer and that the decomposition of the former to H2S and CO2 is prevented by a barrier in excess of 30 kcal mol⁻¹. Therefore, we attempted to prepare carbonothioic-O,O-acid under matrix isolation conditions via high vacuum flash pyrolysis (HVFP) of O,S-di-tert-butyl carbonothioate in analogy to the previously reported successful preparation of carbonic acid. Pyrolysis at 900 °C only produced isobutene, CO2, and tert-buthylthiol, while S-tert-butyl O-hydrogen carbonothioate is the most likely additional product identifiable at 690 °C pyrolysis temperature. Density functional theory computations at the M06-2X/cc-pVTZ level were used to understand the pyrolysis pathways and help determine the leaving group ability in the ester pyrolysis step, and our inability to observe the carbonothioic acids via our chosen pyrolysis path.
- Generation and Characterization of the Phenylthiyl Radical and its Oxidation to the Phenylthiylperoxy and Sulfonylbenzenyl Radical
-
Artur Mardyukov and Peter R. Schreiner*
PCCP 2016, 18, 26161–26165. DOI: 10.1039/C6CP04278CThe phenylthiyl radical (1) was prepared in the gas phase by vacuum flash pyrolysis of allylphenyl sulfide or diphenyl sulfide and isolated in an argon matrix. The hitherto unknown phenylthiyl peroxy radical was synthesized by co-condensation of 1 with molecular oxygen. Irradiation with light of λ = 465 nm led to a rearrangement to the novel phenylsulfonyl radical.
- Forming Stereogenic Centers in Acyclic Systems from Alkynes
-
Roxane Vabre, Biana Island, Claudia Diehl, Peter R. Schreiner, and Ilan Marek*
Angew. Chem. Int. Ed. 2015, 54, 9996–9999. DOI: 10.1002/anie.201504756
Noted as very important paper (top 5% of all Angewandte publications)
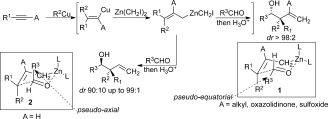
The combined carbometalation/zinc homologation followed by reactions with α-heterosubstituted aldehydes and imines proceed through a chair-like transition structure with the substituent of the incoming aldehyde residue preferentially occupying a pseudo-axial position to avoid the two gauche interactions. The heteroatom in the axial position produces a chelated intermediate (and not a Cornforth–Evans transition structure for α-chloro aldehydes and imines) leading to a face differentiation in the allylation reaction. This method provides access to functionalized products in which three new carbon–carbon bonds and two to three stereogenic centers, including a quaternary one, were created in acyclic systems in a single-pot operation from simple alkynes.
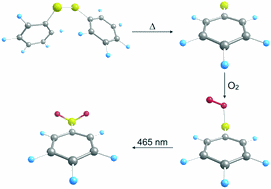
- Reactions of the methylsulfinyl radical [CH3(O)S•] with oxygen (3O2) in solid argon
-
Hans Peter Reisenauer, Jarosław Romański, Grzegorz Mlostoń, Peter R. Schreiner
Chem. Commun. 2015, 51, 10022–10025. DOI: 10.1039/c5cc02168e
Highlight: Holm Petzold, Nachr. Chem. 2015, 63, 760
The atmospherically highly relevant methylsulfinyl radical (CH3(O)S•) readily reacts with molecular triplet oxygen in cryogenic argon matrices containing small amounts of 3O2. Comparison of experimental and computed IR- and UV/Vis spectra, including isotope exchange, show that the initially formed 3O2 adduct has the structure of a peroxyl radical (CH3(O)SOO•), which upon irradiation with UV light isom-erizes to the methylsulfonoxyl radical (CH3SO3•). The latter transforms into the methansulfonic acid radical (•CH2SO3H) by irradiation with visible light. During the matrix photolysis small amounts of SO3 and methyl radical were detected indi-cating competitive direct photodissociation.

- Gas Phase Generation and Matrix Isolation of the Methylsulfonyl Radical CH3SO2• from Allylmethylsulfone
-
Hans-Peter Reisenauer, Jaroslaw Romanski, Peter R. Schreiner, and Grzegorz Mloston
J. Phys. Chem. A 2015, Article ASAP. DOI: 10.1021/jp5036647
The atmospherically highly relevant methylsulfonyl radical (CH3SO2•) was generated by high-vacuum flash pyrolysis (HVFP) of allylmethylsulfone and isolated in an argon matrix at 10 K; the allyl radical formed as the cofragment. Upon thermolysis, the methylsulfonyl radical undergoes partial decomposition, leading to substantial amounts of sulfur dioxide in the matrix. The title compound was characterized through the assignment of eight fundamental IR bands of its CD3 and 13CH3 isotopologues and the excellent agreement with the B3LYP/6-311+G(3df,3pd) computed harmonic vibrational frequencies. The two most intense absorptions were found at 1267.1 and 1067.6 cm–1. In extension of this study S-methyl methanethiosulfonate was found to be another suitable, although less efficient, precursor for the gas-phase generation of the methylsulfonyl radical.
- Gas phase preparation of carbonic acid and its monomethyl ester.
-
Hans Peter Reisenauer, Jan Philipp Wagner, Peter R. Schreiner
Angew. Chem. Int. Ed. 2014, 53, 11766–11771 (“hot paper”), DOI: 10.1002/anie.201406969.
Highlights:
a) Front cover of this issue;
b) Perspective: Götz Bucher and Wolfram Sander Science 2014, 346, 544–545; c) Feature: Carbonic Acid Crystal Forms Identified. Jyllian N. Kemsley C & EN News 2014, 92 (41), 28–29;
d) ChemistryViews: Carbonic Acid – And Yet It Exists!;
e) Innovations Report: Carbonic Acid—And Yet It Exists!;
f) Physorg.com: Preparation and characterization of gas-phase carbonic acid and its monomethyl ester.
 Carbonic acid (H2CO3), an essential molecule of life (e.g., as bicarbonate buffer), has been well characterized in solution and in the solid state, but for a long time, it has eluded its spectral characterization in the gas phase owing to a lack of convenient preparation methods; microwave spectra were recorded only recently. Here we present a novel and general method for the preparation of H2CO3 and its monomethyl ester (CH3OCO2H) through the gas-phase pyrolysis of di-tert-butyl and tert-butyl methyl carbonate, respectively. H2CO3 and CH3OCO2H were trapped in noble-gas matrices at 8 K, and their infrared spectra match those computed at high levels of theory [focal point analysis beyond CCSD(T)/cc-pVQZ] very well. Whereas the spectra also perfectly agree with those of the vapor phase above the β-polymorph of H2CO3, this is not true for the previously reported α-polymorph. Instead, the vapor phase above α-H2CO3 corresponds to CH3OCO2H, which sheds new light on the research that has been conducted on molecular H2CO3 over the last decades.
Carbonic acid (H2CO3), an essential molecule of life (e.g., as bicarbonate buffer), has been well characterized in solution and in the solid state, but for a long time, it has eluded its spectral characterization in the gas phase owing to a lack of convenient preparation methods; microwave spectra were recorded only recently. Here we present a novel and general method for the preparation of H2CO3 and its monomethyl ester (CH3OCO2H) through the gas-phase pyrolysis of di-tert-butyl and tert-butyl methyl carbonate, respectively. H2CO3 and CH3OCO2H were trapped in noble-gas matrices at 8 K, and their infrared spectra match those computed at high levels of theory [focal point analysis beyond CCSD(T)/cc-pVQZ] very well. Whereas the spectra also perfectly agree with those of the vapor phase above the β-polymorph of H2CO3, this is not true for the previously reported α-polymorph. Instead, the vapor phase above α-H2CO3 corresponds to CH3OCO2H, which sheds new light on the research that has been conducted on molecular H2CO3 over the last decades.
- Combined ab initio molecular dynamics and experimental studies show that carbon atom addition to benzene.
-
Michael L. McKee, Hans-Peter Reisenauer, and Peter R. Schreiner,
J. Phys. Chem. A. 2014, 118, 2801–2809. DOI: 10.1021/jp501107b.
Car–Parrinello molecular dynamics was used to explore the reactions between triplet and singlet carbon atoms with benzene. The computations reveal that, in the singlet C atom reaction, products are
 very exothermic where nearly every collision yields a product that is determined by the initial encounter geometry. The singlet C atom reaction does not follow the minimum energy path because the bimolecular reaction is controlled by dynamics (i.e., initial orientation of encounter). On the other hand, in a 10 K solid Ar matrix, ground state C(3P) atoms do tend to follow RRKM kinetics. Thus, ab initio molecular dynamics (AIMD) results indicate that a significant fraction of C–H insertion occurs to form phenylcarbene whereas, in marked contrast to previous theoretical and experimental conclusions, the Ar matrix isolation studies indicate a large fraction of direct cycloheptatetraene formation, without the intermediacy of phenylcarbene. The AIMD calculations are more consistent with vaporized carbon atom experiments where labeling studies indicate the initial formation of phenylcarbene. This underlines that the availability of thermodynamic sinks can completely alter the observed reaction dynamics.
very exothermic where nearly every collision yields a product that is determined by the initial encounter geometry. The singlet C atom reaction does not follow the minimum energy path because the bimolecular reaction is controlled by dynamics (i.e., initial orientation of encounter). On the other hand, in a 10 K solid Ar matrix, ground state C(3P) atoms do tend to follow RRKM kinetics. Thus, ab initio molecular dynamics (AIMD) results indicate that a significant fraction of C–H insertion occurs to form phenylcarbene whereas, in marked contrast to previous theoretical and experimental conclusions, the Ar matrix isolation studies indicate a large fraction of direct cycloheptatetraene formation, without the intermediacy of phenylcarbene. The AIMD calculations are more consistent with vaporized carbon atom experiments where labeling studies indicate the initial formation of phenylcarbene. This underlines that the availability of thermodynamic sinks can completely alter the observed reaction dynamics.
- Matrix Isolation and Spectroscopic Properties of the Methylsulfinyl Radical CH3(O)S•
-
Hans Peter Reisenauer,* Jaroslav Romanski, Grzegorz Mloston,* and Peter R. Schreiner,
Chem. Commun. 2013, 9467–9469. DOI: 10.1039/c3cc45379k
The atmospherically highly relevant methylsulfinyl radical CH3(O)S• was generated thermally under flash pyrolysis conditions and isolated in Ar matrices at 10 K; the allyl radical is a byproduct. CH3(O)S• and its D3- and 13C-isotopologues were characterized through the excellent agreement between experimental and computed IR and UV/Vis spectra.
- Thermolysis of 3,3,5,5-Tetramethyl-1,2,4-triothiolane-1-oxide: Matrix Isolation of the HOSS•-Radical.
-
Hans-Peter Reisenauer,* Grzegorz Mloston,* Jaroslaw Romanski, and Peter R. Schreiner,
Eur. J. Org. Chem. 2012, 3408–3415. DOI: 10.1002/ejoc.201200146
Flash vacuum pyrolysis of 3,3,5,5-tetramethyl-1,2,4-trithiolane 1-oxide performed at 700 °C yields the 1-oxatrisulfan-3-yl radical (HOSS·) along with disulfur monoxide (S2O) and diisopropyl sulfide, which were isolated in argon matrices at 10 K. Upon irradiation with UV light, the 1-oxatrisulfan-3-yl radical undergoes isomerization to the 1-oxatrisulfan-1-yl radical (HSSO·). Both radicals were identified by comparison of their computed and experimental IR and UV/Vis spectra. In addition, density functional theory (DFT) computations offer a plausible explanation of the most likely reaction mechanism, suggesting that the initial step is a 1,3-H shift with simultaneous ring opening. A 1-oxatrisulfane derivative formed thereby undergoes fragmentations via a radical and a competitive concerted pathway leading to the observed final products. The same mechanism also governs the thermal fragmentation of di-tert-butyl disulfide S-oxide. Its pyrolysis at 700 °C affords an analogous set of products, including the 1-oxatrisulfan-3-yl radical (HOSS·) as the key intermediate.
- Conformations and Reactions of Bicyclo[3.2.1]oc-6-en-8-ylidene.
-
Udo H. Brinker, Alexander A. Bespokoev, Hans Peter Reisenauer, and Peter R. Schreiner
J. Org. Chem. 2012, 77, 3800–3807. DOI: 10.1021/jo3001035
![Conformations and Reactions of Bicyclo[3.2.1]oct-6-en-8-ylidene](https://www.uni-giessen.de/de/fbz/fb08/Inst/organische-chemie/agschreiner/research/images/carbenes/ylideneudo/@@images/image-0-71398ec3ce010e59ffd8ad9b31a86e11.jpeg) Bicyclo[3.2.1]oct-6-en-8-ylidene (1) can assume either the conformation of “classical” carbene 1a or that of foiled carbene 1b in which the divalent carbon bends toward the double bond. Oxadiazoline precursors for the generation of 1 were prepared, followed by photochemical and thermal decomposition as well as flash vacuum pyrolysis (FVP) of a tosyl hydrazone sodium salt precursor, to give a number of rearrangement products. Matrix isolation experiments demonstrate the presence of a diazo intermediate and methyl acetate in all photochemical and thermal precursor reactions. The major product from rearrangements of “classical” bridged carbene 1a is bicyclo[3.3.0]octa-1,3-diene as a result of an alkyl shift, while dihydrosemibullvalene formed from a 1,3-C–H insertion. In contrast, thus far unknown strained bicyclo[4.2.0]octa-1,7-diene formed by a vinyl shift in foiled carbene 1b. The experimental results are corroborated by density functional theory (DFT), MP2, and G4 computations.
Bicyclo[3.2.1]oct-6-en-8-ylidene (1) can assume either the conformation of “classical” carbene 1a or that of foiled carbene 1b in which the divalent carbon bends toward the double bond. Oxadiazoline precursors for the generation of 1 were prepared, followed by photochemical and thermal decomposition as well as flash vacuum pyrolysis (FVP) of a tosyl hydrazone sodium salt precursor, to give a number of rearrangement products. Matrix isolation experiments demonstrate the presence of a diazo intermediate and methyl acetate in all photochemical and thermal precursor reactions. The major product from rearrangements of “classical” bridged carbene 1a is bicyclo[3.3.0]octa-1,3-diene as a result of an alkyl shift, while dihydrosemibullvalene formed from a 1,3-C–H insertion. In contrast, thus far unknown strained bicyclo[4.2.0]octa-1,7-diene formed by a vinyl shift in foiled carbene 1b. The experimental results are corroborated by density functional theory (DFT), MP2, and G4 computations.
- Oxathiirane
-
Peter R. Schreiner, Hans Peter Reisenauer, Jaroslaw Romanski, and Grzegorz Mloston
J. Am. Chem. Soc. 2010, 132, 7240–7241. Download
We describe the first preparation of the long sought after parent oxathiirane from sulfine through photochemical rearrangement with light at 313 ± 10 nm in an Ar-matrix at 11 K. Oxathiirane was characterized by the extraordinarily good agreement of experimentally measured and CCSD(T)/cc-pVTZ (unscaled) computed vibrational frequencies both for the perhydrogenated and perdeuterated species. The title molecule is about 10 kcal mol–1 less stable than sulfine, in marked contrast to the isomer energy difference of dioxirane vs. carbonyl oxide (ca. –25 kcal mol–1). This is due to the strong positive polarization (blue potential) vs. the highly electronegative oxygen atom (red). The stability ordering and the relative energy differences of carbonyl vs. thiocarbonyl groups underlines the likely role oxathiiranes play in sulfur transfer reactions.

- Thermal Reactions of Regioisomeric 1,2,4-Trithiolane S-Oxides.
-
Grzegorz Mloston, Jaroslaw Romanski, Michael L. McKee, Hans-Peter Reisenauer, and Peter R. Schreiner
Eur. J. Org. Chem. 2010, 2132–2137. Download
The products of the gas phase pyrolysis of two regioisomeric 1,2,4-trithiolane S-oxides were collected in an argon matrix at 10 K and studied by means of spectroscopic as well as computational methods. Whereas the main products of the pyrolysis of the ‘symmetrical’ S-oxide were identified as thioformaldehyde S-oxide and thioformaldehyde S-sulfide, the ‘non-symmetrical’ S-oxide gave predominantly dithioformic acid, which exists as a mixture of s-cis and s-trans conformers. We present a rationalization of the reaction pathways including density functional theory computations.
- A formal carbon-sulfur triple bond: HCSOH.
-
Peter R. Schreiner, Hans Peter Reisenauer, Jaroslaw Romanski, and Grzegorz Mloston
Angew. Chem. Int. Ed. 2009, 48, 8133–8136. Download; Highlight: Neil Withers Nature Chem. 2009. Link; Robert Berger Nachr. Chem. 2010, 58, 7. Blog
Extremely rare: a CS triple bond can be assigned to HCSOH, a new molecule prepared through a photochemical [1,3]H-shift of sulfine H2C=S=O. But does this formal description agree with analyses on the basis of IR vibrations, bond lengths, bond orders, molecular orbitals, and compliance constants? Such types of molecules challenge and refine our current understanding of chemical bonding.

- Infrared Signatures of the NCCO Radical.
-
Peter R. Schreiner, Hans Peter Reisenauer, Edit Mátyus, Attila G. Császár, Ali Siddiqi, Andrew C. Simmonett, and Wesley D. Allen
Phys. Chem. Chem. Phys. 2009, 11, 10385-10390. Download
The first definitive infrared signatures of the elusive NCCO radical have been measured using a microwave discharge technique combined with low-temperature matrix-isolation spectroscopy, resulting in a consistent set of vibrational assignments for six isotopologues. The infrared spectra of these NCCO isotopologues were concomitantly established by rigorous variational nuclear-motion computations based on a high-level coupled-cluster quartic vibrational force field [ROCCSD(T)/cc-pCVQZ] and cubic dipole field [ROCCSD/cc-pCVTZ]. Our experimental and theoretical results for NCCO overturn the vibrational assignments in a NIST-JANAF compilation and those from a recent two-dimensional cross-spectral correlation analysis. For the parent isotopologue at 11 K in a nitrogen matrix, we find the signature bands n2(CO str.) = 1889.2 cm-1 and n3(CC str.) = 782.0 cm-1. Our variational vibrational computations reveal strong mixing of the n3 stretching fundamental and the n4+n5 bending combination level for all isotopologues. These Fermi resonances manifest a clear breakdown of the simple normal-mode picture of molecular vibrations at low energies.
- Prototypical Triplet Alkyl Phosphonatocarbenes.
-
Adelina Nemirowski, Hans Peter Reisenauer, Jaroslaw Romanski, Grzegorz Mloston, and Peter R. Schreiner,
J. Phys. Chem. A, 2008, 50, 13244. Download
Abstract. The current case study focuses on the generation, identification, and characterization of two representative mono- and disubstituted alkyl phosphonatocarbenes by means of matrix isolation techniques in conjunction with density functional theory [B3LYP/6-311++G(d,p)] and coupled cluster [CCSD(T)/cc-pVXZ, X = D, T] computations. The EPR measurements identify both carbenes as triplet ground-state species with D values of 0.660 and 0.623 cm–1 respectively, exhibiting persistency toward intramolecular reactions (the EPR signal observable in perfluoromethylcyclohexane up to around 70 K for the disubstituted molecule). While the reaction of the carbene center of the conformationally rich tetramethyl bisphosphonatocarbene with the CH bonds of the methyl groups leads to phosphaoxetane at room temperature, its fragmentation via a Wittig-type reaction during high vacuum flash pyrolysis (HVFP) results in dimethyl vinylphosphonate and methyl metaphosphate. The latter has been observed for the first time as an isolated entity.

- Electronic Stabilization of Ground State Triplet Carbenes.
-
Adelina Nemirowski and Peter R. Schreiner, J. Org. Chem. 2007, 72, 9533. Download
Abstract. Based on systematic ab initio (CCSD(T)/cc-pVDZ) studies of substituent effects, we present a concept for the construction of electronically stabilized triplet ground state carbenes with singlet−triplet energy separations (∆EST) exceeding that of methylene. Sterically demanding and conjugating substituents were excluded from the selection of model compounds under investigation, as these either destabilize both the singlet and the triplet states or delocalize unpaired spins away from the carbene carbon. Negative partial charges on the carbene center allow for the prediction of the electronic stabilization of substituted carbenes. To decrease carbene reactivity, we chose b-substituents with strong polar bonds. Among them, highly electronegative elements such as fluorine and oxygen enlarge the ∆EST value with respect to hydrogen, while chlorine does not due to p-orbital participation.
- Dimethoxycarbene: Conformational Analysis of a Reactive Intermediate.
-
Hans Peter Reisenauer, Jaroslaw Romanski, Grzegorz Mloston, and Peter R. Schreiner,
Eur. J. Org. Chem. 2006, 4813. Download
Abstract. Dimethoxycarbene was prepared from an oxadiazoline precursor under high-vacuum flash pyrolysis (HVFP) conditions and was trapped at low temperatures by matrix isolation techniques (Ar, 10 K). The excellent agreement between the computed [CCSD(T)/cc-pVDZ] IR spectrum of the mixture of conformers of dimethoxycarbene and the experimentally measured IR absorptions allows a detailed analysis ofthe conformational preference of dimethoxycarbene. ItsUV spectrum is in agreement with earlier studies and our TD-B3LYP/6-311+G(d,p) computations. The computed [CCSD(T)/cc-pVDZ] rotational profile is rather steep and separates the s-trans,s-trans and s-cis,s-trans conformers by a 16 kcal mol-1 barrier, whilst the s-cis,s-cis conformer is too high-lying to be observable (+17 kcal mol-1). In marked contrast with the gauche,gauche minimum of dimethoxymethane, the s-trans,s-trans conformer of dimethoxycarbene is slightly preferred (0.5 kcal mol-1). The s-cis,s-trans conformer equilibrates at the high temperatures required during HFVP generation and both conformers can be identified in the IR spectrum of the argon matrix at 10 K. The conformational preference is partly due to the minimization of the overall dipole moment in the s-trans,s-trans conformer.
- H–C–SiH3: Direct Generation and Spectroscopic Identification of Ethylidene’s Cousin.
-
Peter R. Schreiner, Hans Peter Reisenauer, Kurt W. Sattelmeyer, and Wesley D. Allen,
J. Am. Chem. Soc. 2005, 127, 12156. Download
Abstract. Ground-state triplet silaethylidene, generated directly by the reaction of 3P carbon atoms with silane under matrix isolation conditions in solid Ar (10–12 K), has been thoroughly characterized by the EPR and IR spectra of both the parent and perdeuterated isotopologs. A theoretical anharmonic vibrational analysis based on a CCSD(T)/cc-pVTZ complete quartic force field gave remarkable agreement with the experimental IR fundamentals, generally within 10 cm–1 and without any empirical scaling of the ab initio frequencies. Silaethylidene exhibits a CS minimum with a H–C–Si angle near 153 °, but the barrier to H–C–Si linearity (C3v symmetry) is only 0.24 kcal mol–1. This minuscule barrier can be surmounted by zero-point vibrations, as evident from the EPR data. The triplet stabilizing effect of the electropositive SiH3 group amounts to about 15 kcal mol–1.