Thermolysis of 3,3,5,5-Tetramethyl-1,2,4-triothiolane-1-oxide: Matrix Isolation of the HOSS•-Radical.
Hans-Peter Reisenauer,* Grzegorz Mloston,* Jaroslaw Romanski, and Peter R. Schreiner,
Eur. J. Org. Chem. 2012, 3408–3415. DOI: 10.1002/ejoc.201200146
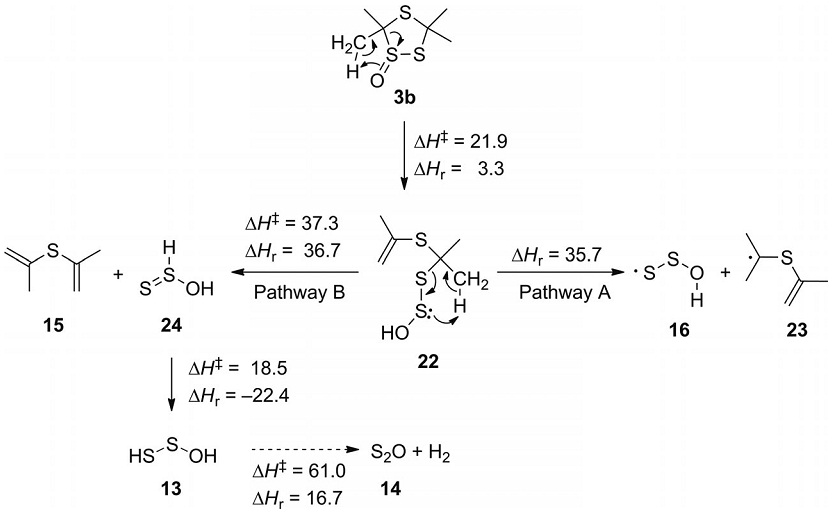
Flash vacuum pyrolysis of 3,3,5,5-tetramethyl-1,2,4-trithiolane 1-oxide performed at 700 °C yields the 1-oxatrisulfan-3-yl radical (HOSS·) along with disulfur monoxide (S2O) and diisopropyl sulfide, which were isolated in argon matrices at 10 K. Upon irradiation with UV light, the 1-oxatrisulfan-3-yl radical undergoes isomerization to the 1-oxatrisulfan-1-yl radical (HSSO·). Both radicals were identified by comparison of their computed and experimental IR and UV/Vis spectra. In addition, density functional theory (DFT) computations offer a plausible explanation of the most likely reaction mechanism, suggesting that the initial step is a 1,3-H shift with simultaneous ring opening. A 1-oxatrisulfane derivative formed thereby undergoes fragmentations via a radical and a competitive concerted pathway leading to the observed final products. The same mechanism also governs the thermal fragmentation of di-tert-butyl disulfide S-oxide. Its pyrolysis at 700 °C affords an analogous set of products, including the 1-oxatrisulfan-3-yl radical (HOSS·) as the key intermediate.
