Publications
40. Facile (3+2) Cycloaddition between an N-Heterocyclic Olefin and Nitrous Oxide at Ambient Conditions
J. Ariai, J. Becker, U. Gellrich European Journal of Organic Chemistry 2024, 27, e202301252.
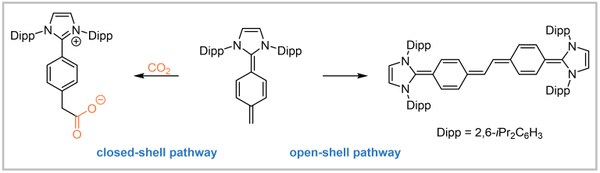
39. An N-Heterocyclic Quinodimethane: A Strong Organic Lewis Base Exhibiting Diradical Reactivity
J. Ariai, M. Ziegler, C. Würtele, U. Gellrich Angewandte Chemie International Edition 2024, 63, e202316720; Angewandte Chemie 2024, 136, e202316720.
38. Synthesis and characterization of a formal 21-electron cobaltocene derivative
S. Takebayashi, J. Ariai, U. Gellrich, S. V. Kartashov, R. R. Fayzullin, H.-B. Kang, T. Yamane, K. Sugisaki, K. Sato Nature Communications 2023, 14, 4979.
Highlighted in Nature Chemistry: A 21-electron cobalt sandwich.
J. Ariai, U. Gellrich Physical Chemistry Chemical Physics 2023, 25, 14005–14015.
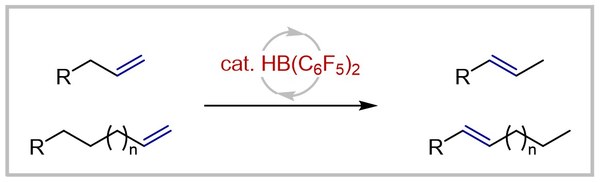
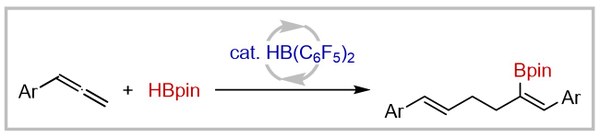
36. Bis(pentafluorophenyl)borane-catalyzed E-selective isomerization of terminal alkenes to internal alkenes (invited contribution to Organic Chemistry Frontiers Emerging Investigator Series and selected as HOT article)
R. S. Phatake, T. Müller, A. Averdunk, U. Gellrich Organic Chemistry Frontiers 2023, 10, 1128–1133.
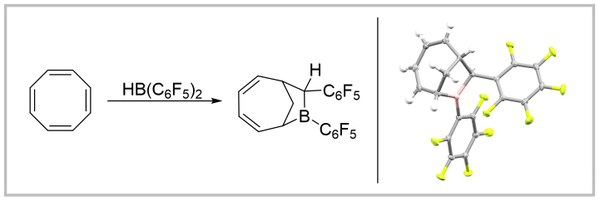
35. Bis(pentafluorophenyl)borane Induced Tandem Hydroboration-Carboboration of Cyclooctatetraene (invited contribution to the special collection dedicated to Prof. Doug Stephan's 70th birthday)
T. Müller, M. Hasenbeck, J. Becker, U. Gellrich Zeitschrift für anorganische und allgemeine Chemie 2023, 649, e202200381.
R. S. Phatake, A. Averdunk, C. Würtele, U. Gellrich ACS Catalysis 2022, 12, 13961–13968.
F. Wech, U. Gellrich ACS Catalysis 2022, 12, 5388–5396.
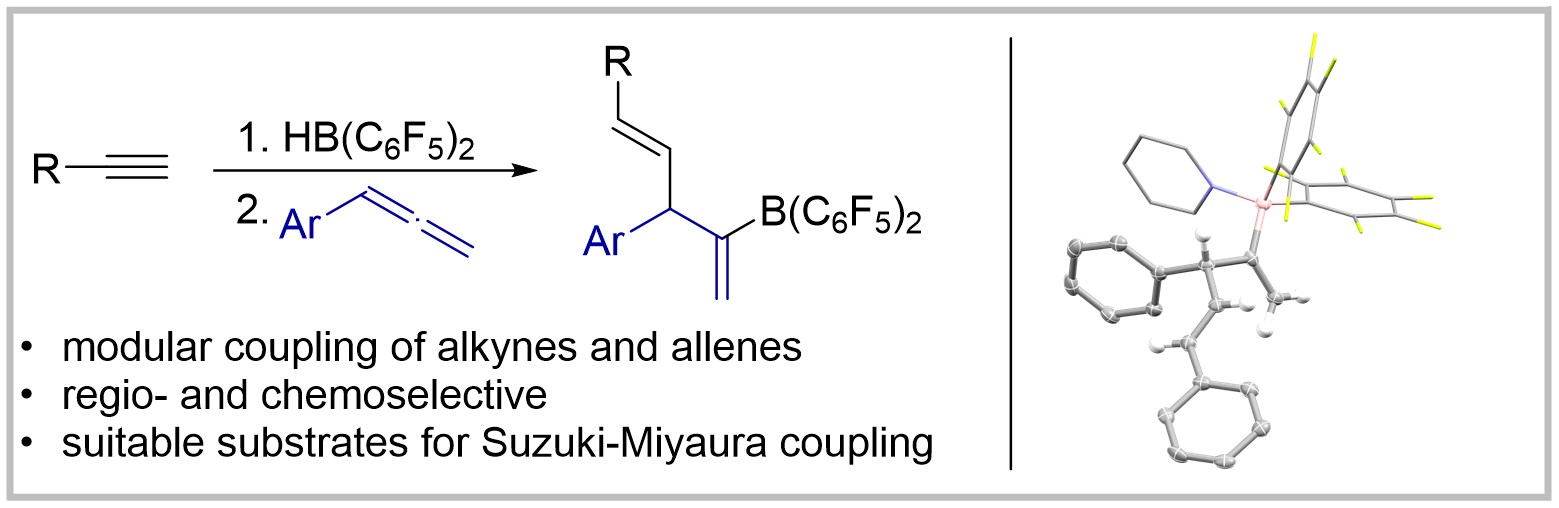
32. 1,2-Carboboration of Arylallenes by In Situ Generated Alkenylboranes for the Synthesis of 1,4-Dienes
A. Averdunk, M. Hasenbeck, T. Müller, J. Becker, U. Gellrich Chemistry—A European Journal 2022, 28, e2022004.
Highlighted in ChemistryViews: 1,2-Carboboration of Arylallenes.
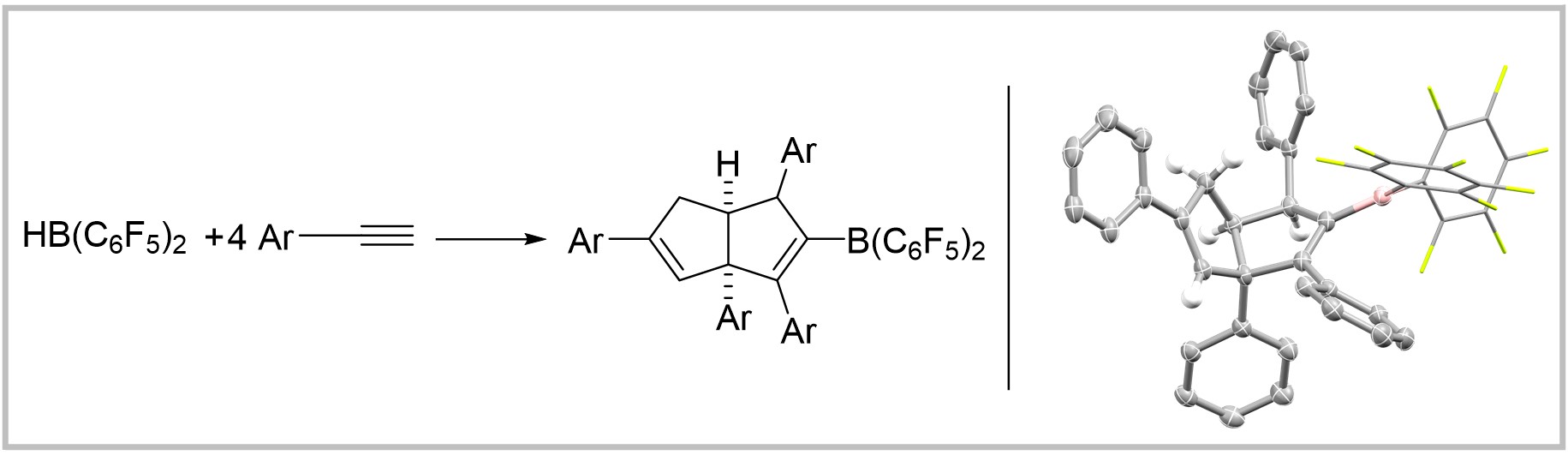
31. Piers' Borane Induced Tetramerization of Arylacetylenes
M. Hasenbeck, T. Müller, A. Averdunk, J. Becker, U. Gellrich, Chemistry—A European Journal 2021, 28, e2021042.
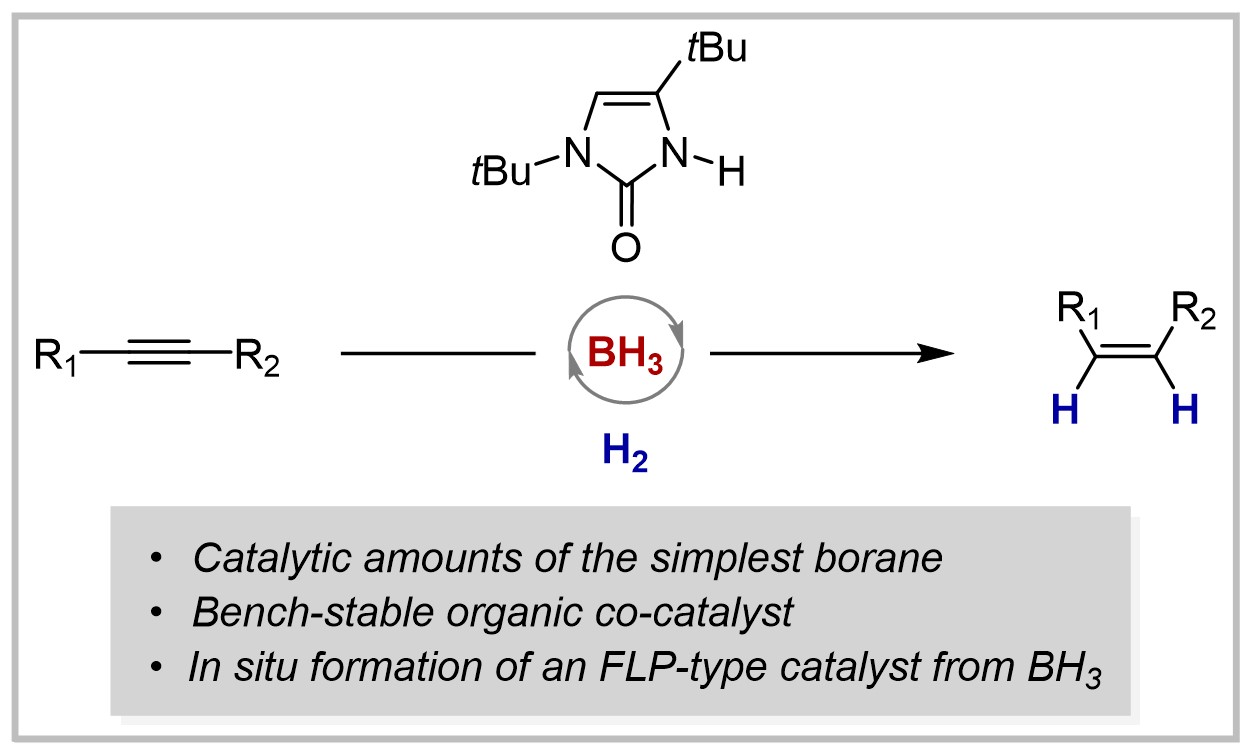
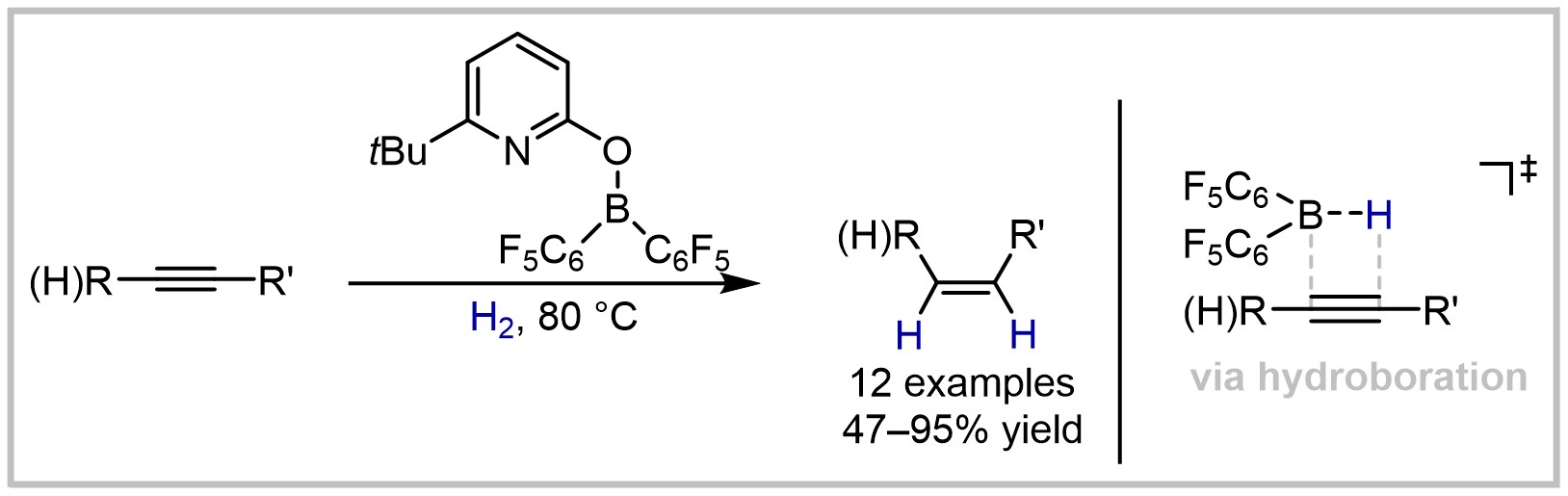
30. Hydrogenation of Olefins, Alkynes, Allenes, and Arenes by Borane-based Frustrated Lewis Pairs (invited contribution to the Bürgenstock Special Section)
F. Wech, U. Gellrich, Synthesis 2021, 54, 3421–3431.
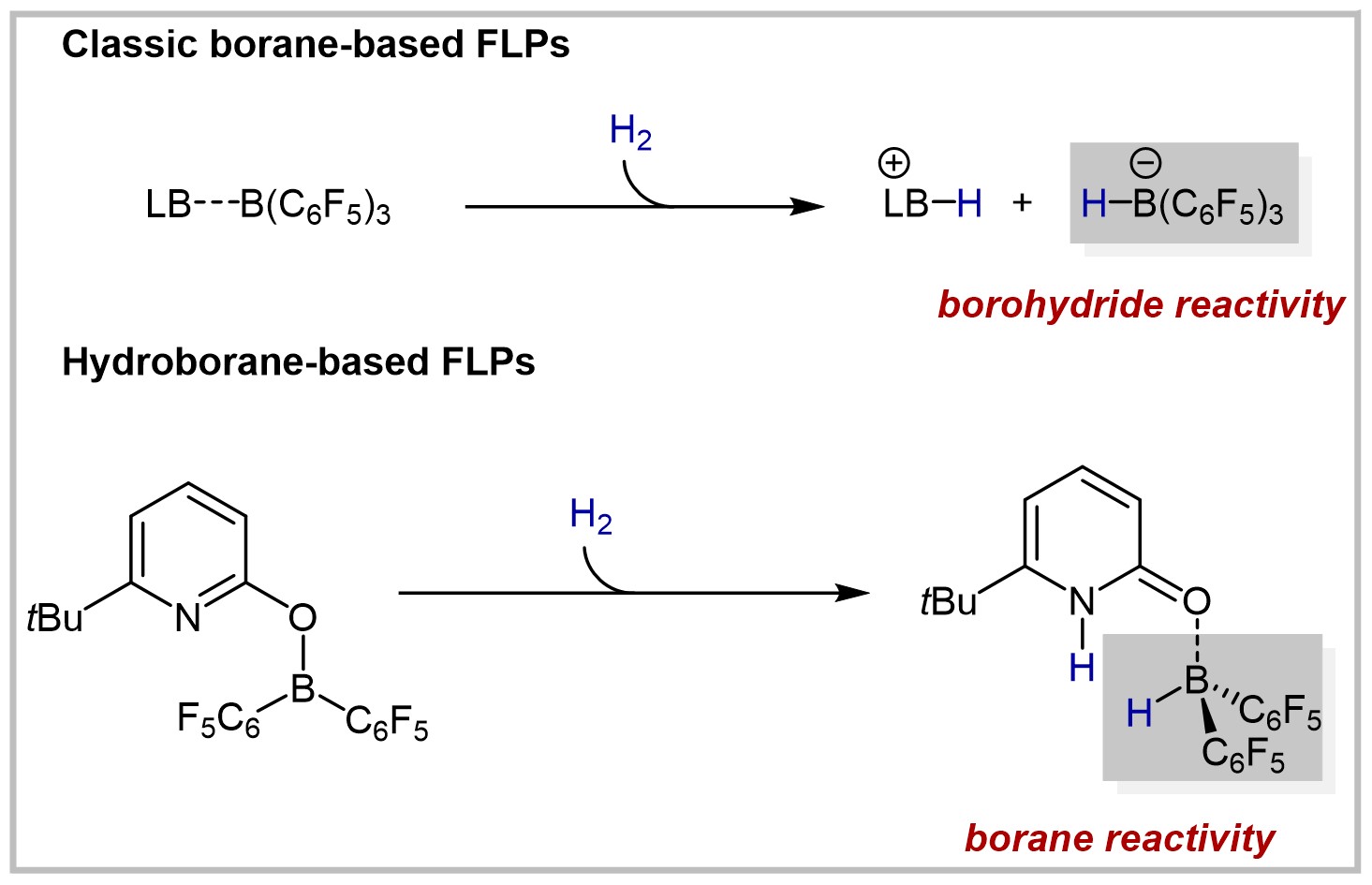
29. Reactions of Allenes with Borane-Based Lewis Acids (invited contribution to the YourJOC Talents and highlighted as Very Important Paper)
U. Gellrich, European Journal of Organic Chemistry 2021, 2021, 4707–4714.
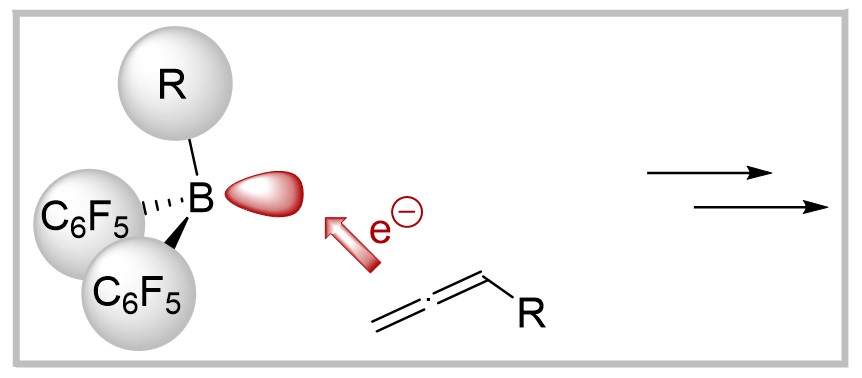
M. Hasenbeck, F. Wech, A. Averdunk, J. Becker, U. Gellrich, Chemical Communications 2021, 57, 5518–5521.
27. Boron‐Ligand Cooperation: The Concept and Applications (invited minireview)
M. Hasenbeck, U. Gellrich, Chemistry—A European Journal 2021, 27, 5615–5626.
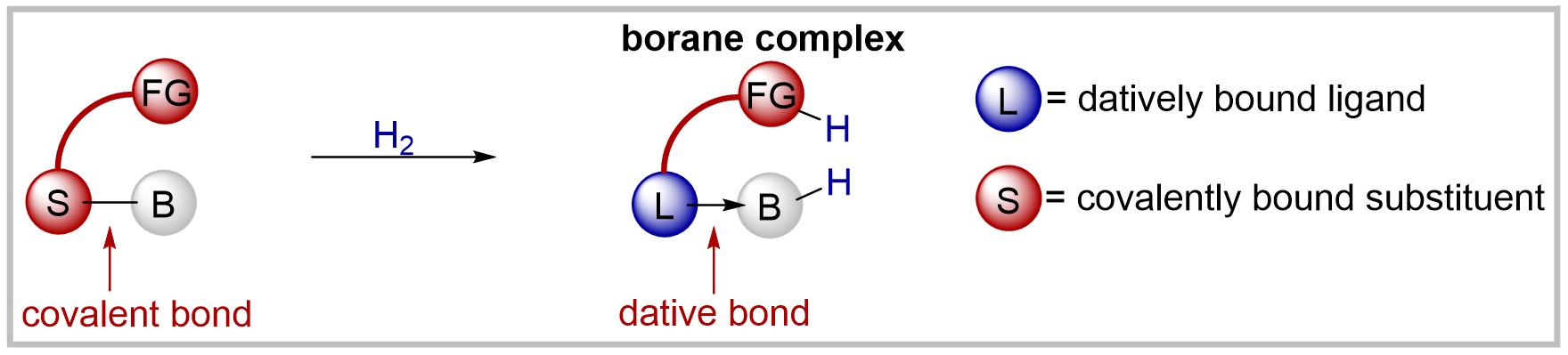
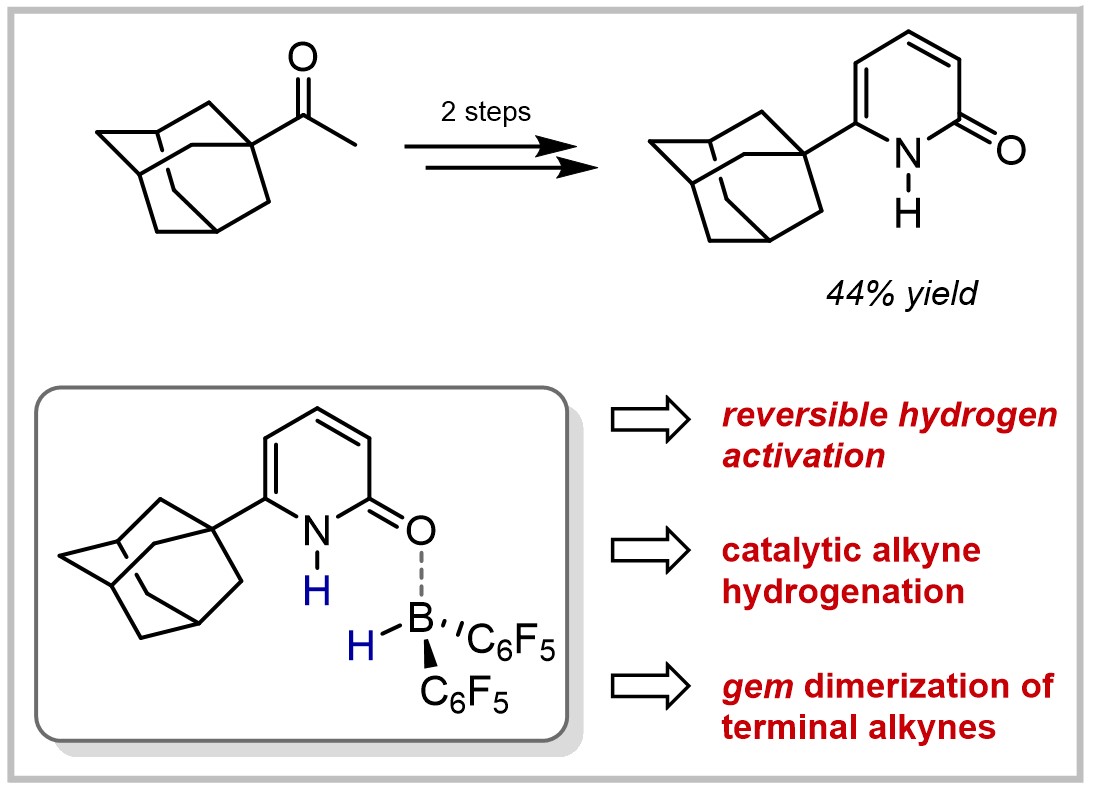
26. Synthesis of 6-Adamantyl-2-pyridone and Reversible Hydrogen Activation by the Corresponding Bis(perfluorophenyl)borane Complex (invited feature article)
F. Wech, T. Müller, J. Becker, U. Gellrich, Synthesis 2021, 53, 666–672.
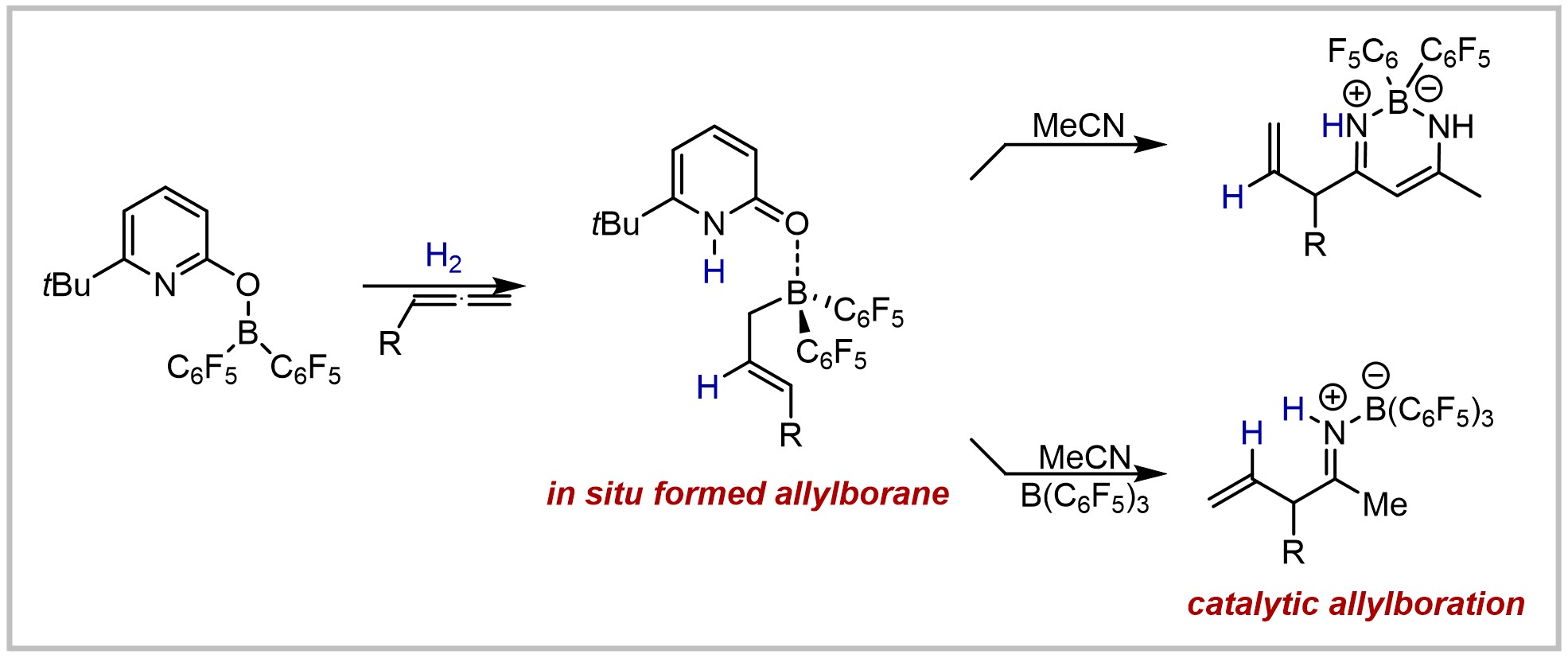
M. Hasenbeck, S. Ahles, A. Averdunk, J. Becker, U. Gellrich, Angewandte Chemie International Edition 2020, 59, 23885–23891; Angewandte Chemie 2020, 132, 24095–24101.
Highlighted in Organic Process Research & Development: Some Items of Interest to Process R&D Chemists and Engineers.
F. Wech, M. Hasenbeck, U. Gellrich, Chemistry—A European Journal 2020, 26, 13445–13450.
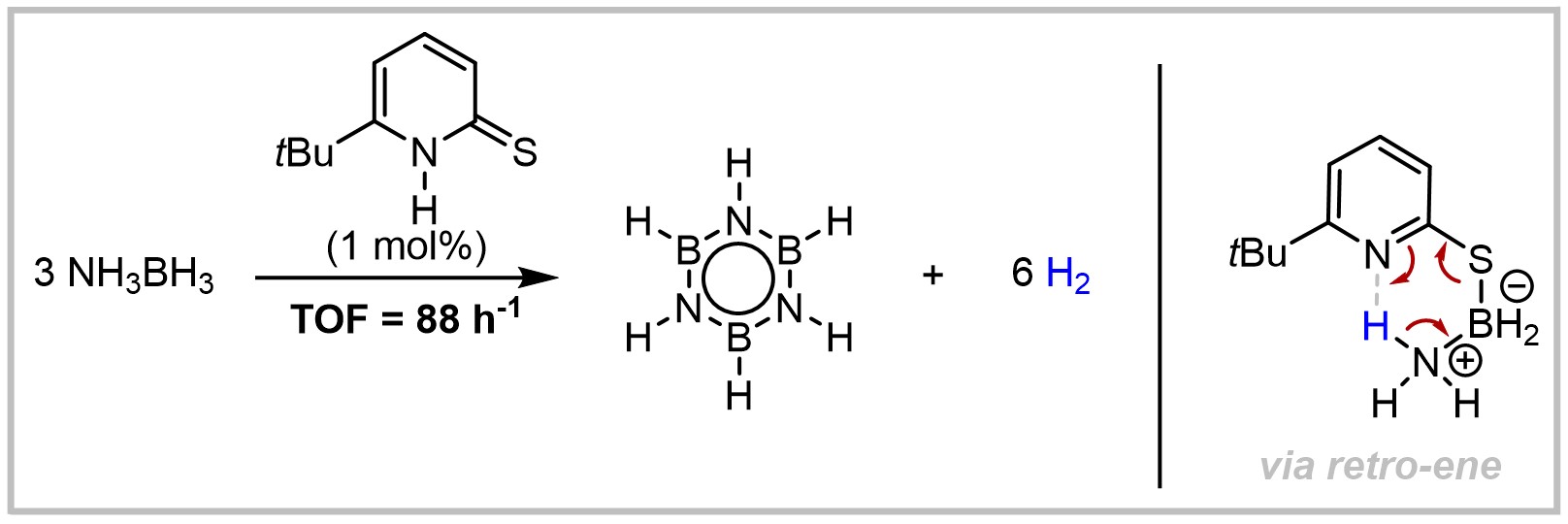
23. Efficient Organocatalytic Dehydrogenation of Ammonia‐Borane
M. Hasenbeck, J. Becker, U. Gellrich, Angewandte Chemie International Edition 2020, 59, 1590–1594; Angewandte Chemie 2020, 132, 1606–1610.
Highlighted in Synfacts: Organocatalysis Meets Hydrogen Storage: Efficient Dehydrogenation of Ammonia-Borane.
Highlighted in Nachrichten aus der Chemie in March 2021.
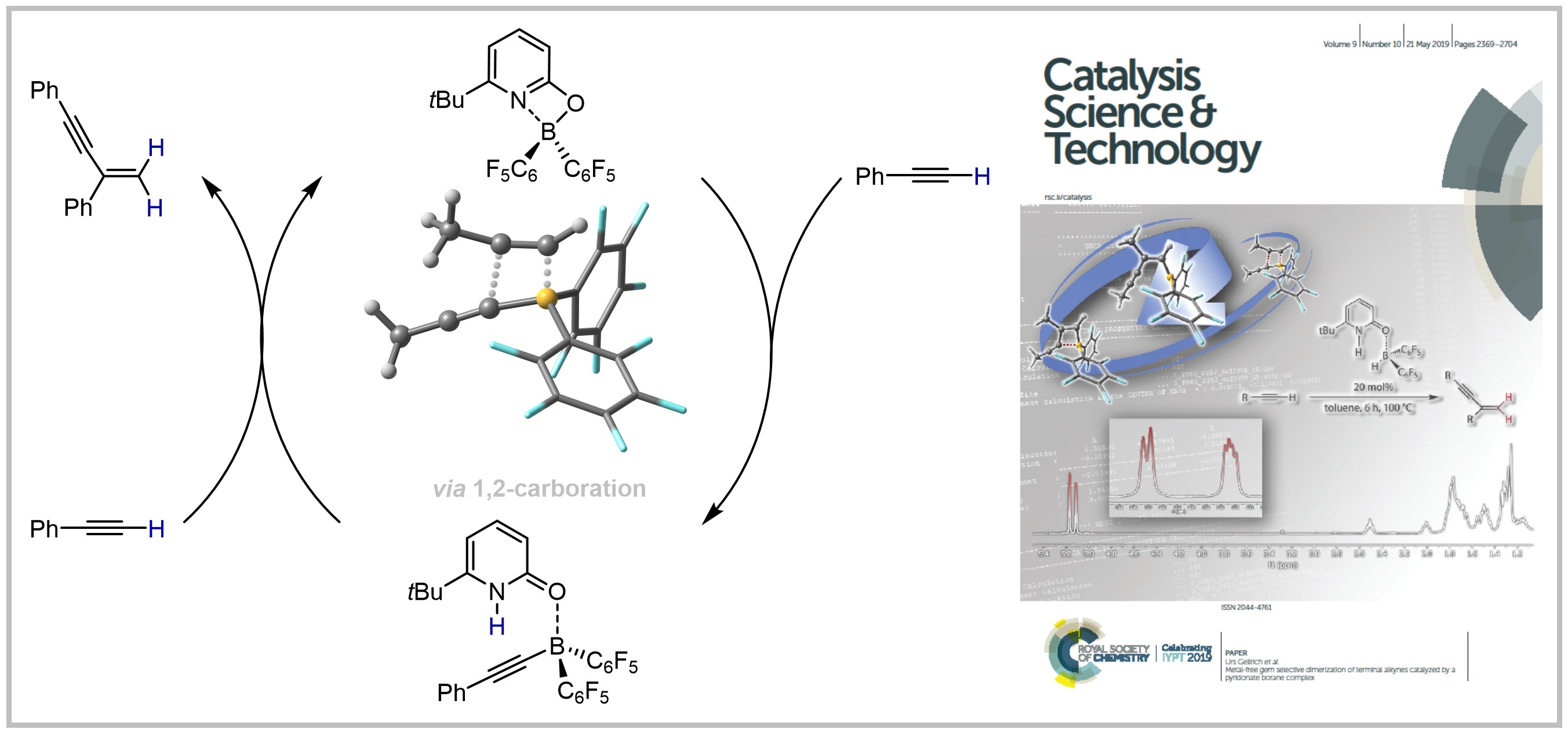
22. Metal-free gem selective Dimerization of Terminal Alkynes catalyzed by a Pyridonate Borane complex
M. Hasenbeck, T. Müller, U. Gellrich, Catalysis Science & Technology 2019, 9, 2438-2444.
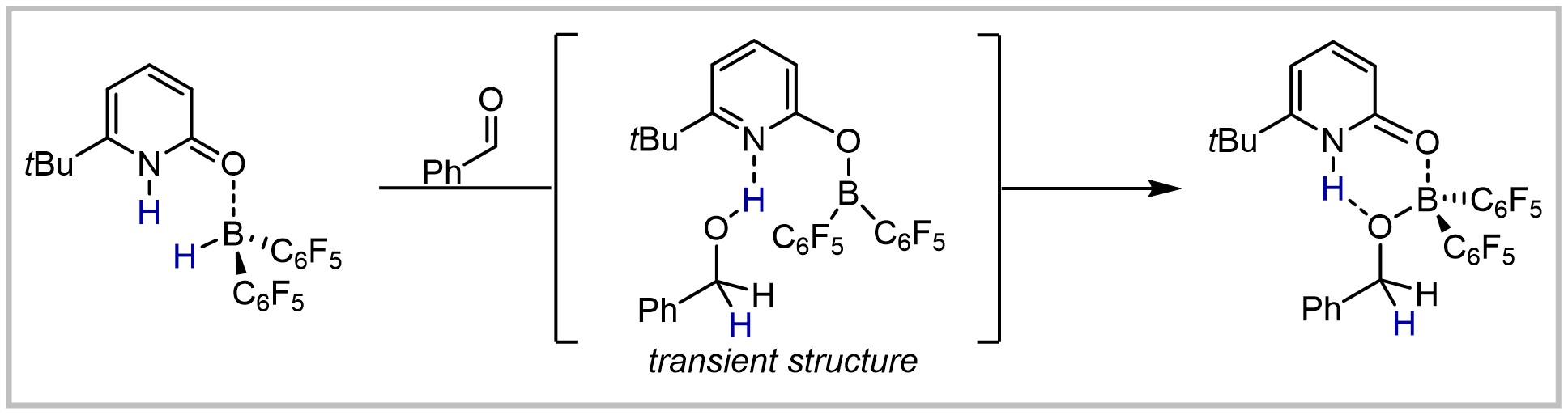
21. Aldehyde Reduction by a Pyridone Borane Complex via Boron-Ligand-Cooperation: Concerted or not?
T. Müller, M. Hasenbeck, J. Becker, U. Gellrich, European Journal of Organic Chemistry 2019, 2019, 451–457.
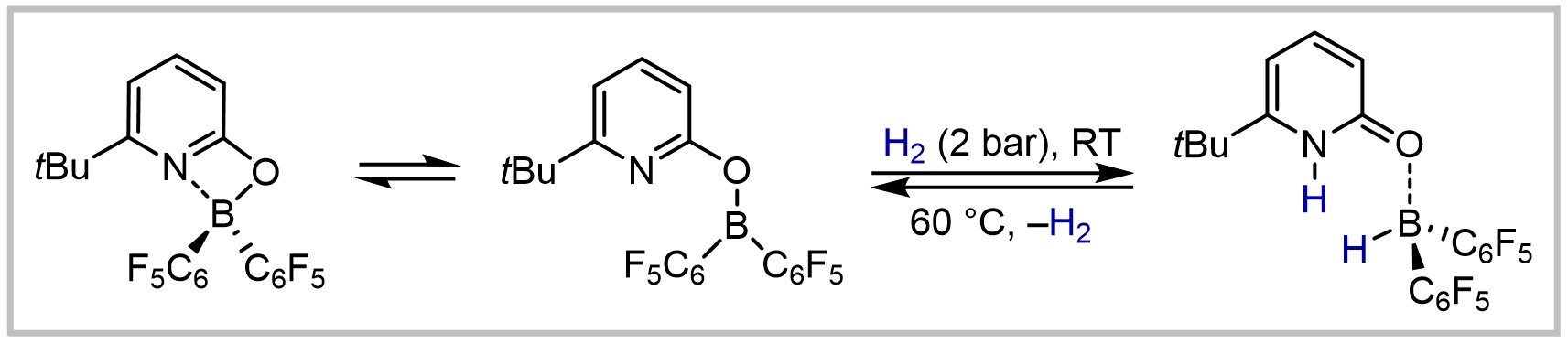
U. Gellrich, Angewandte Chemie International Edition 2018, 57, 4779–4782; Angewandte Chemie 2018, 130, 4869–4872.
PhD and PostDoc
S. Chakraborty, U. Gellrich, Y. Diskin-Posner, G. Leitus, L. Avram, D. Milstein, Angewandte Chemie International Edition 2017, 56, 4229–4233; Angewandte Chemie 2017, 129, 4293–4297.
18. The Ferraquinone–Ferrahydroquinone Couple: Combining Quinonic and Metal-Based Reactivity
A. Dauth, U. Gellrich, Y. Diskin-Posner, Y. Ben-David, D. Milstein, Journal of the American Chemical Society 2017, 139, 2799–2807.
17. Reversible Aromaticity Transfer in a Bora-Cycle: Boron–Ligand Cooperation
U. Gellrich, Y. Diskin-Posner, L. J. W. Shimon, D. Milstein, Journal of the American Chemical Society 2016, 138, 13307–13313.
A. Nerush, M. Vogt, U. Gellrich, G. Leitus, Y. Ben-David, D. Milstein, Journal of the American Chemical Society 2016, 138, 6985–6997.
15. Reductive Cleavage of CO2 by Metal–Ligand-Cooperation Mediated by an Iridium Pincer Complex
M. Feller, U. Gellrich, A. Anaby, Y. Diskin-Posner, D. Milstein, Journal of the American Chemical Society 2016, 138, 6445–6454.
14. Rh chemistry through the eyes of theory
U. Gellrich, T. Koslowski, WIREs Computational Molecular Science 2016, 6, 311–320.
U. Gellrich, J. R. Khusnutdinova, G. M. Leitus, D. Milstein, Journal of the American Chemical Society 2015, 137, 4851–4859.
12. Full kinetic analysis of a rhodium-catalyzed hydroformylation: beyond the rate-limiting step picture
U. Gellrich, T. Koslowski, B. Breit, Catalysis Science & Technology, 2015, 5, 129–133.
B. Breit, U. Gellrich, T. Li, J. M. Lynam, L. M. Milner, N. E. Pridmore, J. M. Slattery, A. C. Whitwood, Dalton Transactions 2014, 43, 11277–11285.
10. Mechanistic Investigations of the Rhodium Catalyzed Propargylic CH Activation
U. Gellrich, A. Meissner, A. Steffani, M. Kähny, H.-J. Drexler, D. Heller, D. A. Plattner, B. Breit, Journal of the American Chemical Society 2014, 136, 1097–1104.
U. Gellrich, D. Himmel, M. Meuwly, B. Breit, Chemistry—A European Journal 2013, 19, 16272–16281.
J. Schaefer, A. Kraft, S. Reininger, G. Santiso-Quinones, D. Himmel, N. Trapp, U. Gellrich, B. Breit, I. Krossing, Chemistry—A European Journal 2013, 19, 12468–12485.
L. Diab, U. Gellrich, B. Breit, Chemical Communications 2013, 49, 9737–9739.
J. Huang, D. Häussinger, U. Gellrich, W. Seiche, B. Breit, M. Meuwly, The Journal of Physical Chemistry B 2012, 116, 14406–14415.
U. Gellrich, W. Seiche, M. Keller, B. Breit, Angewandte Chemie International Edition 2012, 51, 11033 –11038; Angewandte Chemie, 2012, 124, 11195–11200.
4. Enantioselective Titanium(III)-Catalyzed Reductive Cyclization of Ketonitriles
J. Streuff, M. Feurer, P. Bichovski, G. Frey, U. Gellrich, Angewandte Chemie International Edition 2012, 51, 8661–8664; Angewandte Chemie 2012, 124, 8789–8792.
D. Fuchs, G. Rousseau, L. Diab, U. Gellrich, B. Breit, Angewandte Chemie International Edition 2012, 51, 2178–2182; Angewandte Chemie 2012, 124, 2220–2224.
U. Gellrich, J. Huang, W. Seiche, M. Keller, M. Meuwly, B. Breit, Journal of the American Chemical Society 2011, 133, 964–975.
T. Köchner, S. Riedel, A. J. Lehner, H. Scherer, I. Raabe, T. A. Engesser, F. W. Scholz, U. Gellrich, P. Eiden, R. A. Paz Schmidt, D. A. Plattner, I. Krossing, Angewandte Chemie International Edition 2010, 49, 8139 –8143; Angewandte Chemie 2010, 122, 8316–8320.
