Urolithins
Urolithins
Urolithins are intestinal metabolites produced by microbial in vitro fermentation of ellagic acid rich fruits like pomegranate or raspberries.[1-3] Their structure is based on a biaryl system with a central lactone. An example of this key structural motif, 6H-benzo[c]chromen-6-one, is shown below in red.
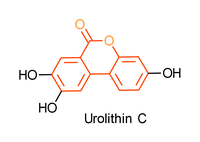
Urolithin derivatives show various very potent biological activities such as anti-tumor, anti-HIV or anti-oxidant activity. Recent studies have shown that the health-promoting effect depends on their biologic activity, which correlates with the number of hydroxyl groups.[2]
The objective of our research is to investigate the synthesis of polyhydroxylated Urolithins.
In the course of our synthetic studies of transition metal catalyzed reactions, we have developed two possible methods for the synthesis of asymmetrical Urolithin derivatives.
One is the intramolecular palladium(0)-catalyzed Heck-like cross coupling reaction, the so called Lactone-Concept.
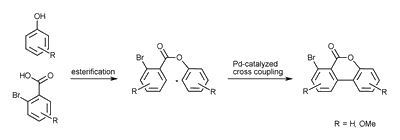
The other is the Cooper (II) catalyzed coupling in one step.
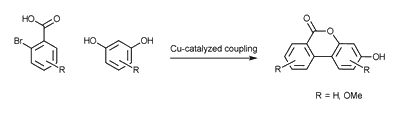
Both methods proved suitable and we isolated various protected Urolithin derivatives.
The final step, the deprotection of the hydroxy-groups, is tedious due to the poor solubility of polyhydroxylated Urolithins in any kind of organic solvent or even water.
[1] J.C. Espín, M. Larrosa, M.T. García-conesa, F. Tomás-Barberán, Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: The Evidence So Far., Evidence-based Complementary and Alternative Medicine 2013, 1-11.
E. Haslam, Natural Polyphenols (Vegetable Tannins) as Drugs : Possible Modes of Action., J. Nat. Prod. 1996, 59, 205-215.
[2] V. Furlanetto et al., Ellagic Acid and Polyhydroxylated Urolithins Are Potent Catalytic Inhibitors of Human Topoisomerase II: An in Vitro Study., J. Agric. Food Chem. 2012, 60, 9162-9170.
[3] R. García-Vilalba, D. Beltrán, J.C. Espín, M.V. Selma, F. A. TomásBarberán, Time Course Production of Urolithins from Ellagic Acid by Human Gut Microbiota, J. Agric. Food Chem. 2013, 61, 8797-8806
| Project-Director: | Prof. Dr. Richard Göttlich |
|---|---|
| Co-Worker: | M.Sc. Dina Shaydulina |
