α-Sulfonpeptide
α-Sulfonopeptides:
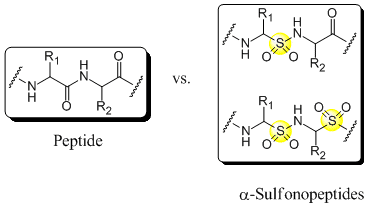
Backbone modified peptides in which at least one of the aminoacids is replaced by one or more α-aminosulfonic acids are called α-sulfonopeptides. Due to the tetrahedral geometry around the sulphur atom those peptidomimetics resemble the intermediate for the peptide bond hydrolysis and therefore they are also called transition state isosteres[1]. Thus α-sulfonopeptides might be, just like the more intensively studied β-sulfonopeptides[2] and vinylogous sulfonopeptides[3], candidates for protease inhibitors with potential therapeutic application. However, the synthesis of α-sulfonopeptides has remained, except for an approach by Curtius Rearrangement[4], elusive due to the susceptibility of α-aminosulfonic acids to decomposition during the activation of the sulfonic acid group[4],[5]. In order to circumvent the activation of the sulfonic acid group we are investigating, among other methods, the electrophilic amination of N-protected α-amino sulfinates[5],[6],as reported by Mulliez et al., further.
[1] R. M. J. Liskamp, D. T. S. Rijkers, J. A. W. Kruijtzer, J. Kemmink, ChemBioChem 2011, 12, 1626–1653
[2] R. de Jong, D. T. S. Rijkers, R. M. J. Liskamp, Helvetica Chimica Acta 2002, 85, 4230–4243
[3] C. Gennari, B. Salom, D. Potenza, A. Williams, Angew. Chem. Int. Ed. Engl. 1994, 33, 2067–2069
[4] S. Paik, E. H. White, Tetrahedron 1996, 52, 5303–5318
[5] F. J. M. Dujols, M. E. Mulliez, J. Org. Chem. 1996, 61, 5648–5649
[6] M. Mulliez, WO 98/04522 A1, 1998
