Molekulare Immunologie
Molekulare Immunologie
- Professur für Immunologie
-
Leitung
Prof. Dr. Andreas Krueger
Schubertstraße 81
35392 Gießen
Tel.: 0641/99-34 25 1
Fax: 0641/99-34 25 9
office@immu.bio.uni-giessen.de
Professur für Immunologie
Leitung
Prof. Dr. Andreas Krueger
Schubertstraße 81
35392 Gießen
Tel.: 0641/99-34 25 1
Fax: 0641/99-34 25 9
office@immu.bio.uni-giessen.de
Forschungsgebiet
Forschung Allgemein
English information below
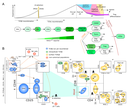
Unser zentrales Forschungsgebiet liegt in den zellulären und molekularen Mechanismen der Entwicklung von Lymphozyten, vor allem T-Zellen, im physiologischen und pathophysiologischen Kontext.
Die Bildung von T-Zellen ist ein dynamischer entwicklungsbiologischer Prozess von erheblicher klinischer Relevanz. Er hängt ab von der Migration von Knochenmark-Vorläuferzellen in den Thymus. Dort erfolgt die Spezifizierung der T-Zell-Linie, die Bildung des T-Zell-Antigen-Rezeptor-Repertoires und die Selektion von T-Zellen, die nicht-funktionelle sowie autoreaktive Zellen eliminiert. Phasen der Rekombination und Selektion sind unterbrochen durch Phasen massiver Proliferation. Aufgrund fortschreitender Involution des Thymus nimmt die Neubildung von T-Zellen mit zunehmendem Alter ab. Diese Abnahme ist ein erhebliches Problem im Rahmen einer hämatopoetischen Stammzelltransplantation. Konditionierungstherapien zerstören das Immunsystem des Patienten, während gleichzeitig die Neubildung von T-Zellen massiv verzögert abläuft. Eine ausgedehnte Phase der Immundefizienz ist die Folge. Die Verbesserung der T-Zell-Regeneration bzw. die „Verjüngung“ des alternden Thymus ist daher von fundamentaler Bedeutung für die Reduktion unerwünschter Wirkungen der Stammzelltransplantation. Darüber hinaus führt eine Fehlregulation des T-Zell-Entwicklungsprogramms häufig zur Ausbildung von Leukämien.
In diesem Kontext verfolgen wir im Wesentlichen drei Arbeitsschwerpunkte:
1) Dynamik der T-Zell-Entwicklung
2) Post-transkriptionelle Regulation der Lymphozytenentwicklung
3) Experimentelle Modelle für humane T-Zell-Entwicklung
Our Research
In order to maintain an efficient line of defense against pathogens, cells of the immune system are continually replenished throughout our life. Rapid regeneration of the immune system after hematopoietic stem cell transplantation (HSCT) is also a major prerequisite to prevent serious complications such as the emergence of fatal viral, bacterial, and fungal infections.
Generation of fresh immune cells is a highly dynamic process and prone to errors, which might ultimately result in development of cancers, such as leukemias, immunodeficiency or autoimmune disease, such as type 1 diabetes, multiple sclerosis, or rheumatoid arthritis. Thus, in depth understanding of the underlying molecular and cellular mechanisms underlying immune cell development are paramount for devising improved therapies for a broad range of immune-system related diseases. Furthermore, development of immune cells undergoes profound changes with increasing age coinciding with declining overall functionality making understanding immune cell development an important goal for medicine in an aging society.
Our laboratory is primarily interested in understanding molecular and cellular mechanisms underlying the formation of T cells. T cells are at the center of the adaptive immune response and direct the immune system to adequately respond to each of the near unlimited variety of pathogens. They are also critical for the formation of immunological memory and, thus, a key target of vaccine development. In addition to these so-called conventional T cells, other “unconventional” T cells exist with a broad range of functions. A certain subset of T cells, so-called regulatory T cells (Treg), help to prevent autoaggression of the immune system. Innate-like T cells, such as invariant Natural Killer T cells (iNKTs) or Mucosa-associated Invariant T cells (MAIT) straddle the realms of innate and adaptive immunity. Little is known about their functions in the host defense against pathogens or immunoregulatory and homeostatic functions.
Specifically, we are working on three aspects of T-cell development.
1) Lymphocyte developmental dynamics
2) Post-transcriptional control of lymphocyte development
3) Models for human T-cell development
Lymphocyte developmental dynamics
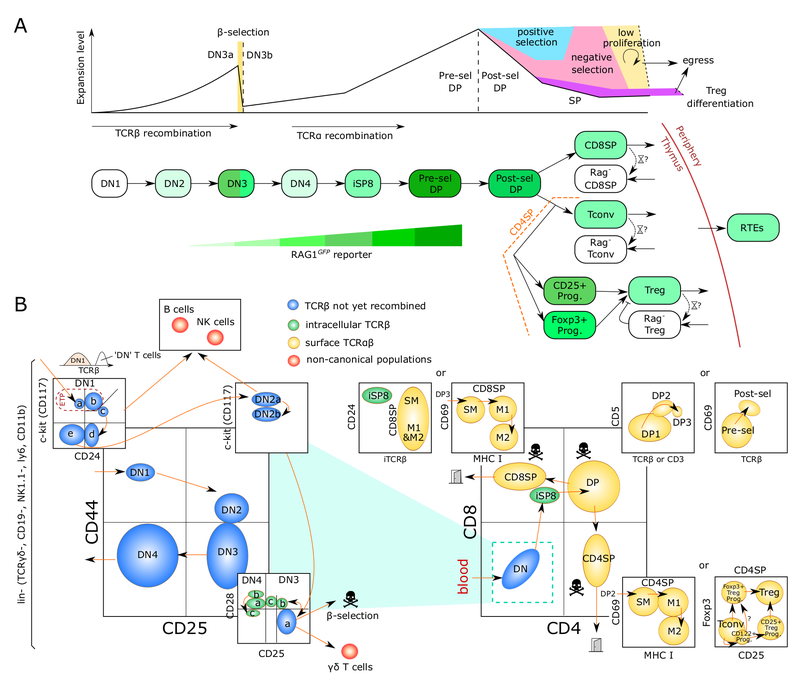
T cells, unlike other blood cells, finalize development in the thymus. We are trying to understand migration of progenitor cells from bone marrow to the thymus in molecular and quantitative terms. To this end, we have recently identified two receptors, CC chemokine receptors 7 and 9, which coordinate colonization of the thymus by progenitor cells. In addition, we have characterized these progenitors by defining minimal common characteristics. We demonstrated that indeed multiple different population act as physiologic thymus seeding progenitors. Recently, we employed a novel approach of multi-congenic fate mapping to quantitate the number of intrathymic niches for thymus seeding progenitors and defined feedback mechanisms that control thymus colonization. Currently, we are developing models of tissue homeostasis in thymus at high resolution. Our experiments have implications for designing pre-transplant conditioning regimens efficiently supporting thymus colonization. Ultimately our experiments are aimed at establishing a complete quantitative spatio-temporal model of early T cell development.
Key publications:
Post-transcriptional control of lymphocyte development
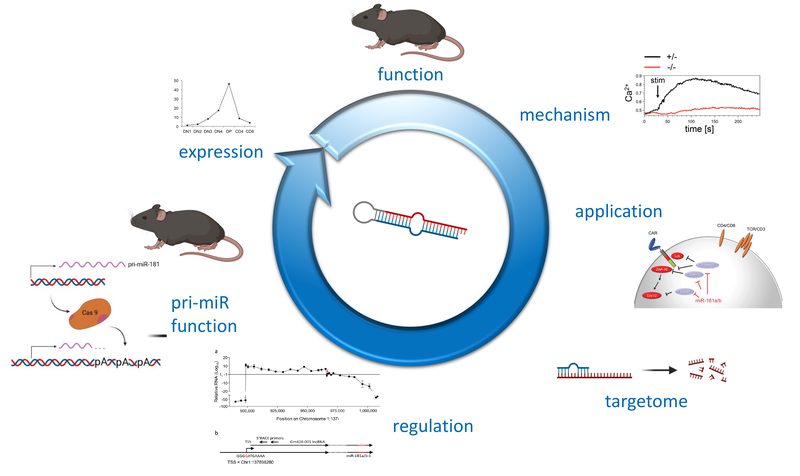
Non-coding (nc)RNAs, such as microRNAs have emerged as a novel layer of post-transcriptional gene regulation. Our laboratory is interest how a progenitor cell’s decision to become a T cell or another lymphoid or non-lymphoid cell type is controlled by ncRNA. To this end, we have conducted microRNA gene expression screens at lineage decision checkpoints and we have begun to establish regulatory networks comprising both, microRNAs and transcriptional regulators. In order to assess the function of microRNAs identified in these screens in vivo we employ classical gene targeting as well as CRISPR/Cas9-mediated deletion. We have shown that deletion of microRNA miR-181a/b-1 causes a profound defect in the generation of unconventional T cells. Furthermore, we have recently demonstrated that a cluster of microRNAs, miR-17~92 also termed (oncomiR-1 because of its function as an oncogene), is critical for early T-cell development. Ultimately, our experiments aim at integrating post-transcriptional gene regulation in a broader gene regulatory context. Understanding post-transcriptional gene regulatory programs will help to better understand molecular mechanisms of tumor formation and may result in the identification of novel therapeutic targets.
Key publications:
Krueger A, Łyszkiewicz M, Heissmeyer V (2022). Post-transcriptional control of T-cell development in the thymus. Immunol Lett 247:1-12.
Grewers Z, Krueger A (2020). MicroRNA miR-181 - A Rheostat for TCR Signaling in Thymic Selection and Peripheral T-Cell Function. Int J Mol Sci. 21(17):6200.
Łyszkiewicz M, Winter SJ, Witzlau K, Föhse L, Brownlie R, Puchałka J, Verheyden NA, Kunze-Schumacher H, Imelmann E, Blume J, Raha S, Sekiya T, Yoshimura A, Frueh JT, Ullrich E, Huehn J, Weiss S, Gutierrez MG, Prinz I, Zamoyska R, Ziętara N*, Krueger A* (2019). miR-181a/b-1 controls thymic selection of Treg cells and tunes their suppressive capacity. PLoS Biol 17(3):e2006716. * Equal contribution.
Winter SJ, Kunze-Schumacher H, Imelmann I, Grewers Z, Osthues T, Krueger A (2019). MicroRNA miR-181a/b-1 controls MAIT cell development. Immunol Cell Biol. 97(2):190-202.
Ziętara N, Łyszkiewicz M, Witzlau K, Naumann R, Hurwitz R, Langemeier J, Bohne J, Sandrock I, Ballmaier M, Weiss S, Prinz I*, and Krueger A* (2013). Critical role for miR-181a/b-1 in agonist selection of invariant natural killer T cells. Proc. Natl. Acad. Sci. USA 110:7407-7412. * Equal contribution.
Models for human T-cell development
Mice with a human immune system constitute excellent models to further our understanding of the development and function of the human immune system, especially with regard to human pathology. However, current models need to be improved to overcome cross-species barriers. To this end, we have generated a BAC-transgenic mouse expressing human IL-7 under control of endogenous gene regulatory elements. Crossed onto the NSGW41 background, this model permits more efficient T-cell development with physiological ratios of B and T cells in the periphery and a diverse TCR repertoire. Strikingly, this model, termed NSGW41hIL7, permitted regeneration of mucosal immunity. This mouse model is well suited for studying human immune regeneration as well as infectious diseases of human specific pathogens and therefore provides an excellent platform for a broad range of potential collaborations.
Key publication:
Coppin E, Sundarasetty BS, Rahmig S, Blume, Verheyden NA, Bahlmann F, Ravens S, Schubert U, Schmid J, Ludwig S, Geissler K, Guntinas-Lichius O, von Kaisenberg C, Groten T, Platz A, Naumann R, Ludwig B, Prinz I, Waskow C*, Krueger A* (2021). Enhanced differentiation of functional human T cells in NSGW41 mice with tissue-specific expression of human interleukin-7. Leukemia, 35(12):356. * Equal contribution
Publications
Publications
2024
2023
Oncoimmunology. 2023 Dec 27;13(1):2296712. doi: 10.1080/2162402X.2023.2296712
2022
Post-transcriptional control of T-cell development in the thymus.
Krueger A, Łyszkiewicz M, Heissmeyer V.
Immunol Lett. 2022 Jul;247:1-12. doi: 10.1016/j.imlet.2022.04.009.
2021
Leukemia. 2021 May 11.
Entropy (Basel). 2021 Apr 8;23(4):437.
2020
Sci Rep. 2020 Dec 8;10(1):21438.
The Role of MicroRNAs in Development and Function of Regulatory T Cells - Lessons for a Better Understanding of MicroRNA Biology.
Kunze-Schumacher H, Krueger A.
Front Immunol. 2020 Sep 9;11:2185.
MicroRNA miR-181-A Rheostat for TCR Signaling in Thymic Selection and Peripheral T-Cell Function.
Grewers Z, Krueger A.
Int J Mol Sci. 2020 Aug 27;21(17):6200.
Enhanced differentiation of functional human T cells in NSGW41 mice with tissue-specific expression of human interleukin-7
Emilie Coppin, Bala Sai Sundarasetty, Susann Rahmig, Jonas Blume, Nikita A. Verheyden, Franz Bahlmann, Sarina Ravens, Undine Schubert, Janine Schmid, Stefan Ludwig, Constantin von Kaisenberg, Alexander Platz, Ronald Naumann, Barbara Ludwig, Immo Prinz, Claudia Waskow, Andreas Krueger
bioRxiv 2020.04.24.060319.
Witkowski M, Witkowski M, Saffarzadeh M, Friebel J, Tabaraie T, Ta Bao L, Chakraborty A, Dörner A, Stratmann B, Tschoepe D, Winter SJ, Krueger A, Ruf W, Landmesser U,
Cardiovasc Diabetol. 2020 Feb 17;19(1):20.
MicroRNA-181a regulates IFN-γ expression in effector CD8+ T cell differentiation.
Amado T, Amorim A, Enguita FJ, Romero PV, Inácio D, de Miranda MP, Winter SJ, Simas JP, Krueger A, Schmolka N, Silva-Santos B, Gomes AQ.
J Mol Med (Berl). 2020 Feb;98(2):309-320.
Magnetic Bead-Based Enrichment of Murine MAIT Cells.
Winter SJ, Krueger A.
Methods Mol Biol. 2020;2098:299-305.
2019
Immunol Cell Biol. 2019; 97(2):190-202.
Eur J Immunol. 2019; 49(1):121-132.
Front Immunol. 2019;10:497.
PLoS Biol. 2019;17(3):e2006716.
Mol Cell Biol. 2019;39(6).
Chimeric antigen receptor-induced BCL11B suppression propagates NK-like cell development.
Maluski M, Ghosh A, Herbst J, Scholl V, Baumann R, Huehn J, Geffers R, Meyer J, Maul H, Eiz-Vesper B, Krueger A, Schambach A, van den Brink MR, Sauer MG.
J Clin Invest. 2019 Sep 3. pii: 126350.
Cossarizza A et al.
Development of Unconventional T Cells Controlled by MicroRNA.
Winter SJ, Krueger A.
Front Immunol. 2019 Oct 23;10:2520.
2018
Decreased production of class-switched antibodies in neonatal B cells is associated with increased expression of miR-181b.
Glaesener S, Jaenke C, Habener A, Geffers R, Hagendorff P, Witzlau K, Imelmann E, Krueger A, Meyer-Bahlburg A.
PLoS One. 2018;13(2):e0192230.
Thymus Colonization: Who, How, How Many?
Krueger A.
Arch Immunol Ther Exp (Warsz). 2018;66(2):81-88.
MicroRNA in T-Cell Development and T-Cell Mediated Acute Graft-Versus-Host Disease.
Koenecke C, Krueger A.
Front. Immunol., 07 May 2018.
miRNA miR-21 Is Largely Dispensable for Intrathymic T-Cell Development.
Kunze-Schumacher H, Winter SJ, Imelmann E, Krueger A.
Front. Immunol. 2018;9:2497.
Genetic models reveal origin, persistence and non-redundant functions of IL-17-producing γδ T cells.
Sandrock I, Reinhardt A, Ravens S, Binz C, Wilharm A, Martins J, Oberdörfer L, Tan L, Lienenklaus S, Zhang B, Naumann R, Zhuang Y, Krueger A, Förster R, Prinz I.
J Exp Med. 2018;215(12):3006-3018.
2017
T Cell Development by the Numbers.
Krueger A, Ziętara N, Łyszkiewicz M.
Trends Immunol. 2017;38:128-139.
2016
Overexpression of Vα14Jα18 TCR promotes development of iNKT cells in the absence of miR-181a/b-1.
Blume J, Zur Lage S, Witzlau K, Georgiev H, Weiss S, Łyszkiewicz M, Ziȩtara N, Krueger A.
Immunol Cell Biol. 2016;94:741-6.
Establishing a murine xenograft-model for long-term analysis of factors inducing chromosomal instability in myelodysplastic syndrome: Pitfalls and successes.
Salari A, Thomay K, Himmler K, Vajen B, Schienke A, Hagedorn M, Ebersold J, Kreipe HH, Krüger A, Schambach A, Schlegelberger B, Göhring G.
Cancer Genet. 2016;209:258-66.
miR-181a Expression in Donor T Cells Modulates Graft-versus-Host Disease after Allogeneic Bone Marrow Transplantation.
Lee CW, Wohlan K, Dallmann I, Förster R, Ganser A, Krueger A, Scherr M, Eder M, Koenecke C.
J Immunol. 2016;196:3927-34.
2015
MicroRNA-181a/b-1 is not required for innate γδNKT effector cell development.
Sandrock I, Ziętara N, Łyszkiewicz M, Oberdörfer L, Witzlau K, Krueger A*, Prinz I*.
PLoS One. 2015;10:e0145010.
* Equal contribution.
Responsiveness of developing T cells to IL-7 signals is sustained by miR-17∼92.
Regelin M, Blume J, Pommerencke J, Vakilzadeh R, Witzlau K, Łyszkiewicz M, Ziętara N, Saran N, Schambach A, Krueger A.
J Immunol. 2015;195:4832-40.
Multicongenic fate mapping quantification of dynamics of thymus colonization.
Ziętara N, Łyszkiewicz M, Puchałka J, Witzlau K, Förster R, Pabst O, Prinz I, Krueger A.
J Exp Med. 2015; 212:1589-1601.
Limited niche availability suppresses murine intrathymic dendritic-cell development from non-committed progenitors.
Łyszkiewicz M, Ziętara N, Föhse L, Puchałka J, Diestelhorst J, Witzlau J, Prinz I, Schambach A,
Krueger A.
Blood. 2015;125:457-64.
2014
Resident CD4+ T cells accumulate in lymphoid organs after prolonged antigen exposure.
Ugur M, Schulz O, Menon MB, Krueger A, Pabst O.
Nat Commun. 2014;5:4821. doi:10.1038/ncomms5821.
B-cell modulation of dendritic-cell function: signals from the far side.
Ziętara N, Łyszkiewicz M, Krueger A*, Weiss S*.
Eur J Immunol. 2014;44:23-32.
*Co-corresponding authors.
S/T phosphorylation of DLL1 is required for full ligand activity in vitro but dispensable for DLL1 function in vivo during embryonic patterning and marginal zone B cell development.
Braune EB, Schuster-Gossler K, Lyszkiewicz M, Serth K, Preusse K, Madlung J, Macek B, Krueger A, Gossler A.
Mol Cell Biol. 2014;34:1221-33.
Hypertrophy of infected Peyer's patches arises from global, interferon-receptor, and CD69-independent shutdown of lymphocyte egress.
Schulz O, Ugur M, Friedrichsen M, Radulovic K, Niess JH, Jalkanen S, Krueger A, Pabst O.
Mucosal Immunol. 2014;7:892-904.
2013
Critical role for miR-181a/b-1 in agonist selection of invariant natural killer T cells.
Ziętara N, Łyszkiewicz M, Witzlau K, Naumann R, Hurwitz R, Langemeier J, Bohne J, Sandrock I, Ballmaier M, Weiss S, Prinz I*, and Krueger A*.
Proc. Natl. Acad. Sci. USA. 2013;110:7407-7412.
* Equal contribution.
Immunoglobulins drive terminal maturation of splenic dendritic cells.
Ziętara N, Łyszkiewicz M, Puchałka J, Gutierrez MG, Pei G, Lienenklaus S, Hobeika E, Reth, M, Martins Dos Santos VAP, Krueger A*, and Weiss S*.
Proc. Natl. Acad. Sci. USA. 2013;110:2282-2287.
* Equal contribution.
2012
Development of interleukin-17 producing <gamma><delta> T cells is restricted to a functional embryonic wave.
Haas JD, Ravens S, Düber S, Chennupati V, Föhse L, Oberdörfer L, Naumann R, Weiß S, Krueger A, Förster R, Prinz I.
Immunity. 2012;37:48-59.
Extra-thymic physiological T lineage progenitor activity is exclusively confined to cells expressing either CD127, CD90, or high levels of CD117.
Saran N, Pommerencke J, Witzlau K, Regelin M, Krueger A.
PLoS One. 2012;7:e30864.
ICOS-dependent stimulation of NKT cells by marginal zone B cells.
Ziętara N, Łyszkiewicz M, Krueger A, and Weiss S.
Eur J Immunol. 2011;41:3125-3134.
Expression of miRNAs miR-133b and miR-206 in the Il17a/f locus is co-regulated with IL-17 production in ab and gd T cells.
Haas JD, Nistala K, Petermann F, Saran N, Chennupati V, Schmitz S, Korn T, Wedderburn LR, Förster R, Krueger A*, Prinz I*.
PLoS One. 2011;6:e20171.
* Equal contribution.
A missing link in thymic dendritic cell development.
Krueger A.
Eur J Immunol. 2011;41:2145-7.
Enforced expression of miR-125b affects myelopoiesis by targeting multiple signalling pathways.
Surdziel E, Cabanski M, Dallmann I, Łyszkiewicz M, Krueger A, Ganser A, Scherr M, Eder M.
Blood. 2011;117:4338-48.
Chemokine receptor CX3CR1 promotes dendritic cell development under steady-state conditions.
Łyszkiewicz M, Witzlau K, Pommerencke J, Krueger A.
Eur J Immunol. 2011;41:1256-65.
2010
CC chemokine receptor (CCR) 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus.
Krueger A*, Willenzon S, Łyszkiewicz M, Kremmer E, Förster R.
Blood. 2010;115:1906-12.
* Corresponding author.
Multiple extra-thymic precursors contribute to T cell development with different kinetics.
Saran N, Łyszkiewicz M, Pommerencke J, Witzlau K, Vakilzadeh R, Ballmaier M, von Boehmer H, Krueger A.
Blood. 2010;115:1137-44.
2009
T cell receptor-instructed alphabeta versus gammadelta lineage commitment revealed by single-cell analysis.
Kreslavsky T, Garbe AI, Krueger A, von Boehmer H.
J Exp Med. 2008;205:1173-86.
2000-2008
T cell receptor-instructed alphabeta versus gammadelta lineage commitment revealed by single-cell analysis.
Kreslavsky T, Garbe AI, Krueger A, von Boehmer H.
J Exp Med. 2008;205:1173-86.
Identification of a T lineage-committed progenitor in adult blood.
Krueger A, von Boehmer H.
Immunity. 2007;26:105-16.
Dynamic visualization of thrombopoiesis within bone marrow.
Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner DD, Graf T, Italiano JE, Shivdasani RA, von Andrian UH.
Science. 2007;317:1767-70.
Phenotypic plasticity of T cell progenitors upon exposure to Notch ligands.
Krueger A*, Garbe AI, von Boehmer H.
J Exp Med. 2006;203:1977-84.
* Corresponding author.
HTLV-1 Tax protects against CD95-mediated apoptosis by induction of the cellular FLICE-inhibitory protein (c-FLIP).
Krueger A*, Fas SC*, Giaisi M, Bleumink M, Merling A, Stumpf C, Baumann S, Holtkotte D, Bosch V, Krammer PH, Li-Weber M.
Blood. 2006;107:3933-9.
* Equal contribution.
The role of CAP3 in CD95 signaling: new insights into the mechanism of procaspase-8 activation.
Golks A, Brenner D, Schmitz I, Watzl C, Krueger A, Krammer PH, Lavrik IN.
Cell Death Differ. 2006;13:489-98.
Differential synergy of Notch and T cell receptor signaling determines alphabeta versus gammadelta lineage fate.
Garbe AI*, Krueger A*, Gounari F, Zuniga-Pflucker JC, von Boehmer H.
J Exp Med. 2006;203:1579-90.
* Equal contribution.
In vitro generated human memory-like T cells are CD95 type II cells and resistant towards CD95-mediated apoptosis.
Fas SC, Baumann S, Krueger A, Frey CR, Schulze-Bergkamen H, Brenner D, Stumpf C, Kappes K, Krammer PH.
Eur J Immunol. 2006;36:2894-903.
c-Myc mediates pre-TCR-induced proliferation but not developmental progression.
Dose M, Khan I, Guo Z, Kovalovsky D, Krueger A, von Boehmer H, Khazaie K, Gounari F.
Blood. 2006;108:2669-77.
FasL (CD95L/APO-1L) resistance of neurons mediated by phosphatidylinositol 3-kinase-Akt/protein kinase B-dependent expression of lifeguard/neuronal membrane protein 35. Beier CP, Wischhusen J, Gleichmann M, Gerhardt E, Pekanovic A, Krueger A, Taylor V, Suter U, Krammer PH, Endres M, Weller M, Schulz JB.
J Neurosci. 2005;25:6765-74.
Glucocorticoids inhibit activation-induced cell death (AICD) via direct DNA-dependent repression of the CD95 ligand gene by a glucocorticoid receptor dimer.
Baumann S, Dostert A, Novac N, Bauer A, Schmid W, Fas SC, Krueger A, Heinzel T, Kirchhoff S, Schütz G, Krammer PH.
Blood. 2005;106:617-25.
Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt.
Schulze-Bergkamen H, Brenner D, Krueger A, Suess D, Fas SC, Frey CR, Dax A, Zink D, Büchler P, Müller M, Krammer PH.
Hepatology. 2004;39:645-54.
Resistance of short term activated T cells to CD95-mediated apoptosis correlates with de novo protein synthesis of c-FLIPshort.
Schmitz I, Weyd H, Krueger A, Baumann S, Fas SC, Krammer PH, Kirchhoff S.
J Immunol. 2004;172:2194-200.
Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs.
Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC, Krueger A, Weigand MA, Grosse-Wilde A, Stremmel W, Krammer PH, Walczak H.
Cell Death Differ. 2004;11:S86-96.
HDAC inhibitors trigger apoptosis in HPV-positive cells by inducing the E2F-p73 pathway.
Finzer P, Krueger A, Stöhr M, Brenner D, Soto U, Kuntzen C, Krammer PH, Rösl F.
Oncogene. 2004;23:4807-17.
Suramin inhibits death receptor-induced apoptosis in vitro and fulminant apoptotic liver damage in mice.
Eichhorst ST*, Krueger A*, Müerköster S, Fas SC, Golks A, Gruetzner U, Schubert L, Opelz C, Bilzer M, Gerbes AL, Krammer PH.
Nat Med. 2004;10:602-9.
* Equal contribution.
An IL-2-dependent switch between CD95 signaling pathways sensitizes primary human T cells toward CD95-mediated activation-induced cell death.
Schmitz I*, Krueger A*, Baumann S, Schulze-Bergkamen H, Krammer PH, Kirchhoff S.
J Immunol. 2003;171:2930-6.
* Equal contribution.
The role of CD95 in the regulation of peripheral T-cell apoptosis.
Krueger A, Fas SC, Baumann S, Krammer PH.
Immunol Rev. 2003;193:58-69.
The active caspase-8 heterotetramer is formed at the CD95 DISC.
Lavrik IN, Krueger A, Schmitz I, Baumann S, Weyd H, Krammer PH, Kirchhoff S.
Cell Death Differ. 2003;10:144-5.
An unexpected role for FosB in activation-induced cell death of T cells.
Baumann S, Hess J, Eichhorst ST, Krueger A, Angel P, Krammer PH, Kirchhoff S.
Oncogene. 2003;22:1333-9.
Specificity of anti-human CD95 (APO-1/Fas) antibodies.
Schmitz I*, Krueger A*, Baumann S, Kirchhoff S, Krammer PH.
Biochem Biophys Res Commun. 2002;297:459-62.
* Equal contribution.
Viral IFN-regulatory factors inhibit activation-induced cell death via two positive regulatory IFN-regulatory factor 1-dependent domains in the CD95 ligand promoter.
Kirchhoff S, Sebens T, Baumann S, Krueger A, Zawatzky R, Li-Weber M, Meinl E, Neipel F, Fleckenstein B, Krammer PH.
J Immunol. 2002;168:1226-34.
Regulation of T cell apoptosis during the immune response.
Baumann S, Krueger A, Kirchhoff S, Krammer PH.
Curr Mol Med. 2002;2:257-72.
Glutathione dependence of caspase-8 activation at the death-inducing signaling complex.
Hentze H, Schmitz I, Latta M, Krueger A, Krammer PH, Wendel A.
J Biol Chem. 2002;277:5588-95.
FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis.
Krueger A, Baumann S, Krammer PH, Kirchhoff S.
Mol Cell Biol. 2001;21:8247-54.
Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex.
Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S.
J Biol Chem. 2001;276:20633-40.
Alternative splicing of caspase-8 mRNA during differentiation of human leukocytes.
Eckhart L., Henry M, Santos-Beneit AM, Schmitz I, Krueger A, Fischer H, Bach J, Ban J, Kirchhoff S, Krammer PH, Mollinedo F, Tschachler E.
Biochem Biophys Res Commun. 2001;289:777-81.
TCR/CD3 Restimulation und CD28 Kostimulation induzieren die Expression von c‑FLIPshort und verhindern CD95 vermittelte Apoptose in T-Zellen.
Kirchhoff S, Krueger A, Baumann S, Krammer PH.
Immunologie Aktuell. 2001;1:117-8. (German).
CD95/CD95 Ligand (Fas/FasL, APO-1/APO-1L).
Krammer PH, Krueger A.
In John Wiley and Sons, I. (ed.). 2001. Wiley Encyclopedia of Molecular Medicine.
Induction of apoptosis in 9-nitrocamptothecin-treated DU145 human prostate carcinoma cells correlates with de novo synthesis of CD95 and CD95 ligand and down-regulation of c-FLIP(short).
Chatterjee D, Schmitz I, Krueger A, Yeung K, Kirchhoff S, Krammer PH, Peter ME, Wyche JH, Pantazis P.
Cancer Res. 2001;61:7148-54.
TCR-mediated up-regulation of c-FLIPshort correlates with resistance toward CD95-mediated apoptosis by blocking death-inducing signaling complex activity.
Kirchhoff S, Müller WW, Krueger A, Schmitz I, Krammer PH.
J Immunol. 2000;165:6293-300.