Functional characterization of ovary-preferentially or -specifically expressed genes in Schistosoma mansoni
Schistosomes have a complex life cycle. Among the remarkable features of schistosome biology is that the induction and maintenance of gonad differentiation in the female depends on a constant pairing contact with the male. This is a prerequisite for egg production. The sexual maturation of the schistosome female is reversible. Upon separation from the male partner, the female stops egg production because the gonads degenerate1, 2.
The scientific focus of my project is the exceptional reproductive biology of schistosomes. Gonad differentiation in the paired female leads to large morphological changes but also to substantial alterations on gene expression in both genders, especially in the female3, 4. This finally leads to the production of composite eggs, which is a complex process (Fig. 1). The maturation of schistosome eggs is classified into five stages based on the relation of the embryo and the eggshell size (Vogel and Prata´s classic staging system)5, 6. In more recent works, the classical Vogel and Prata's staging system was extended with new data generated by histological and whole-mount techniques7.
In paired schistosomes, protein kinase receptors serve as essential signal transduction molecules with regard to the control of differentiation and reproduction processes8-10. Comparative transcriptome studies in adult schistosomes and their (isolated) gonads revealed that 243 (testis) and 3600 (ovary) genes in the gonads are regulated in a pairing-dependent manner4. Some identified but so far uncharacterized receptors comprise potential opsin, retinoid, and thyroid hormone receptors, which are preferentially or specifically transcribed in the ovary of a paired female4. Further analyses over the entire life cycle showed that many of these receptors exhibit differential expression over the life stages, and they are abundantly transcribed in the gonads and/or the egg stage11. This indicates biological importance, which needs to be elucidated. To gain a better understanding of the role of these receptors in developmental processes, the aim of this project is to functionally characterize selected receptors. This will be experimentally determined by knock-down experiments using RNAi, inhibitor studies, interaction studies, and by the establishment of knock-out models.
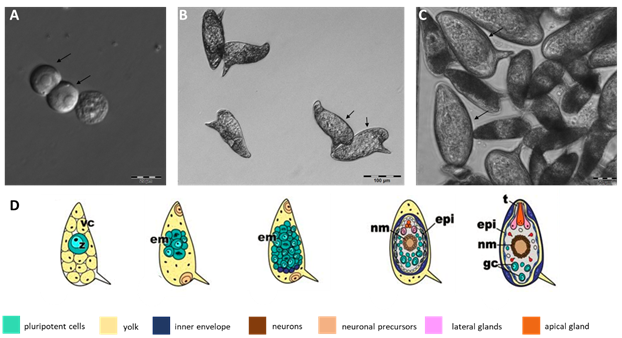 | |
|---|---|
| Fig. 1 Egg development of Schistosoma mansoni from the fertilized zygote to the mature embryo. A, fertilized oocytes (zygotes), phase-I eggs according to Vogel and Prata's classification, obtained as individual cells separated from the surrounding vitelline cells (scale bar 20 µm)5-7. B, phase III eggs, which are characterized by the increase in size of the embryo compared to the egg itself (scale bar: 100 µm). C, phase V eggs (arrows) in comparison to phase III eggs (scale bar: 50 µm). Phase V eggs contain the mature embryo, which leaves the egg as miracidium, a process induced by phototaxis and by changing the osmotic pressure of the surrounding environment. In D, the different developmental stages of the S. mansoni egg are shown, modified according to Jurberg et al. 20097. The development of the egg, according to Vogel and Prata's phase I to V, is shown (from left to right). vc, vitelline cells; z, zygote; em, embryo; nm, neural cells; epi, epidermis; t, terebratorium; gc, terminal cells. | |
1Kunz W (2001) Schistosome male-female interaction: induction of germ-cell differentiation. Trends Parasitol. 17(5):227-31
2Grevelding CG (2004) Schistosoma. Curr Biol. 24;14(14):RS545
3Grevelding CG, Sommer G, Kunz W (1997) Female-specific gene expression in Schistosoma mansoni is regulated by pairing. Parasitology. 115 (Pt 6):635-4
4Lu Z, Sessler F, Holroyd N, Hahnel S, Quack T, Berriman M, Grevelding CG (2016) Schistosome sex matters: a deep view into gonadspecific and pairing-dependent transcriptomes reveals a complex gender interplay. Sci Rep. 6, S. 31150
5Vogel H (1942) Über Entwicklung, Lebensdauer und Tod der Eier vom Bilharzia japonica im Wirtsgewebe. Dtsch Tropenmed Ztsch. 46:57–91
6Prata A (1957) Biópsia retal na esquistossomose mansoni — bases e aplicações no diagnóstico e tratamento. Serviço Nacional de Educação Sanitária-Ministério da Saúde, Rio de Janeiro
7Jurberg AD, Goncalves T, Costa TA, de Mattos AC, Pascarelli BM, de Manso PP, Riberio-Alves M, Pelajo-Machado M, Peralta JM, Coelho PM, Lenzi HL (2009) The embryonic development of Schistosoma mansoni eggs: proposal for a new staging system. Dev Genes Evol. 219(5):219-34
8Beckmann S, Buro C, Dissous C, Hirzmann J, Grevelding CG (2010) The Syk kinase SmTK4 of Schistosoma mansoni is involved in the regulation of spermatogenesis and oogenesis. PLoS Pathog. 6 (2), e1000769
9Gelmedin V, Morel M, Hahnel S, Cailliau K, Dissous C, Grevelding CG (2017) Evidence for integrin - venus kinase receptor 1 alliance in the ovary of Schistosoma mansoni females controlling cell survival. PLoS Pathog. 23;13(1):e1006147
10Morel M, Vanderstraete M, Hahnel S, Grevelding CG, Dissous C. (2014) Receptor tyrosine kinases and schistosome reproduction: new targets for chemotherapy. Front Genet. 18;5:238
11Lu Z, Zhang Y, Berriman M (2018) A web portal for gene expression across all life stages of Schistosoma mansoni. BioRxiv. 308213
