AG Schmitz

copyright: private
Introduction
Our group investigates the molecular mechanisms allowing the transmission of upstream signals to changes in gene expression and chromatin organisation. We study these processes using the NF-κB transcription factor system and the family of stress-regulated homeodomain-interacting protein kinases (HIPKs) as model systems. We investigate how external signals lead to the activation of protein kinases, which then transmit their signals into the nucleus where they regulate transcription factors and epigenetic events. These studies employ a large array of biochemical and cell biological in vitro and cell culture experiments, but also use clinical samples and model organisms for the validation of our findings. The NF-κB and HIPK signal transmission systems are important regulators of the stress response and cell proliferation and are thus found to be dysregulated in a variety of proliferative diseases such as cancer or fibrosis. We are always interested in applications of prospective PhD students or postdocs who are willing to join a hard-working and competitive team with a friendly atmosphere.
copyright: private
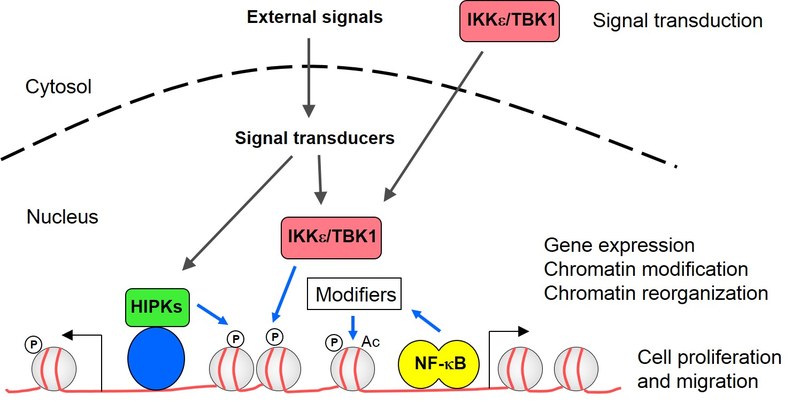
Research
1) The first focus of our research is centered on NF-κB. This transcription factor contributes to the mounting of an effective immune response, but is also involved in the regulation of cell migration, proliferation and apoptosis. The implication of NF-κB in central biological processes and its extraordinary connectivity to other signaling pathways raise a need for highly controlled regulation of NF-κB activity at several levels. The so-called noncanonical IKK kinases IKKε and TBK1 function to trigger the activity of various proinflammatory transcription factors including the NF-κB subunit p65 and IRFs. The analysis of the noncanonical IKKs and their adaptor proteins is of special relevance for the lab, as their overexpression in pathophysiological conditions triggers oncogenic transformation of breast cancer cells and mediates chemoresistance of non-small cell lung cancer and HER2+ breast cancer cells. Mechanistically, overexpression of noncanonical IKKs triggers the expression of anti-apoptotic signaling, stimulates cell proliferation and also allows PI3K-independent direct phosphorylation of the pro-survival kinase AKT. In addition, the constitutive activity of noncanonical IKKs allows for autocrine cytokine circuits that fuel tumor cell growth and cell migration.
NF-κB activity is also regulated within the nucleus. In a collaboration with the group of Michael Kracht (Giessen) we use genome-wide analyses to clarify the contribution of cofactors and upstream kinases for the chromatin recruitment of NF-κB p65 and chromatin modification. We also investigate the genome-wide recruitment of posttranslationally modified NF-κB p65 and a DNA-binding mutant to reveal their physiological function for transcriptional activation and chromatin association. We test the functional consequences of a stimulus-induced conformational switch of NF-κB p65 using a recently identified conformation-specific antibody. These experiments will allow conclusions on the significance of posttranslational modifications, context-specific conformation and DNA-binding for p65 functions, allowing an improved understanding of the molecular mechanisms underlying the plasticity of NF-κB-dependent gene regulation in healthy and diseased cells.
2) The second focus is on the HIPK family of serine/threonine kinases. They function as integrators of a wide variety of stress signals. A number of conditions representing precarious situations such as DNA damage, hypoxia, reactive oxygen intermediates and metabolic stress affect the function of HIPKs. The kinases function as integrators for these stress signals and feed them into many different downstream effector pathways that serve to cope with these precarious situations. Their central role as signaling hubs with the ability to shape many downstream effector pathways frequently implies them in proliferative diseases such as cancer or fibrosis. Using genome-wide assays we study how HIPKs are anchored at specific sites on the genome and how they reprogram gene expression. We have identified chromatin binding sites for a member of the HIPK family using ChIP-Seq and found that the majority of the binding motifs overlaps with a sequence recognized by a ubiquitously expressed transcription factor. The relative contribution of this transcription factor for chromatin attachment of the kinase and the functional relevance for HIPK-regulated gene expression and modification of neighbouring chromatin regions are under investigation. We also study the contribution of these kinases for the coupling of transcription initiation, elongation and the further processing/degradation of the resulting transcripts. This will be of special relevance in certain brain tumors which are addicted to the constitutive activity of HIPKs. As kinases can be targeted by small molecule inhibitors, the development of specific and reversible HIPK inhibitors is a further goal of the lab.

copyright: wordcloud.com
Quantitative display of the words contained in the abstracts of our papers in the last 10 years.
Publications you can find in PubMed.
We participate in these research consortia: SENESCENCE2030 - CA23119, CPI, GRK2573/2
Here is a list of our alumni.
