Reactive Intermediates
Singlet carbenes incorporating a divalent carbon atom (R–C–R’) have grown from laboratory curiosities and theoreticians’ pet peeves into reagents in the growing field of stable carbene chemistry.
Reactive Intermediates1
Singlet carbenes incorporating a divalent carbon atom (R–C–R’) have grown from laboratory curiosities and theoreticians’ pet peeves into reagents in the growing field of stable carbene chemistry. Still, the experimental characterization of many simple yet fundamentally important carbenes (e.g., hydroxycarbenes, alkyl carbenes, etc.) is hampered by their high reactivity or lack of precursors. Hydroxycarbenes have been an unknown class of compounds until 2008, when our group reported the synthesis and characterization of hydroxymethylene (H–C–OH), whose preparation has been challenging organic chemists for more than 80 years. The reaction of hydroxycarbene with formaldehyde would be a source of simple sugars (the so-called “formose reaction” in the origin of life theory). Considerable efforts are ongoing to understand the formation and distribution of simple organics in extraterrestrial environments, and the examination of the structures and reactivities of prototypes such as hydroxycarbene may also provide glimpses of the prebiotic earth.
Current work:
Gas phase preparation of carbonic acid and its monomethyl ester.
Hans Peter Reisenauer, Jan Philipp Wagner, Peter R. Schreiner Angew. Chem. Int. Ed. 2014, 53, 11766–11771 (“hot paper”). DOI: 10.1002/ange.201406969.
Highlights: a) Front cover of this issue; b) Perspective: Götz Bucher and Wolfram Sander Science 2014, 346, 544–545; c) Feature: Carbonic Acid Crystal Forms Identified. Jyllian N. Kemsley C & EN News 2014, 92 (41), 28–29; d)
ChemistryViews: Carbonic Acid – And Yet It Exists!; e) Innovations Report: Carbonic Acid—And Yet It Exists!; f) Physorg.com: Preparation and characterization of gas-phase carbonic acid and its monomethyl ester.
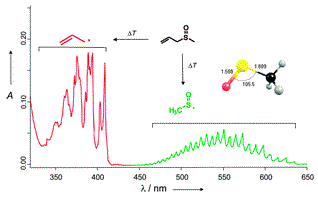
Matrix Isolation and Spectroscopic Properties of the Methylsulfinyl Radical CH3(O)S•. Hans Peter Reisenauer,* Jaroslav Romanski, Grzegorz Mloston,* and Peter R. Schreiner, Chem. Commun. 2013, 9467–9469. DOI: 10.1039/c3cc45379k
Thermolysis of 3,3,5,5-Tetramethyl-1,2,4-triothiolane-1-oxide: Matrix Isolation of the HOSS•-Radical. Hans-Peter Reisenauer,* Grzegorz Mloston,* Jaroslaw Romanski, and Peter R. Schreiner, Eur. J. Org. Chem. 2012, 3408–3415. DOI: 10.1002/ejoc.201200146
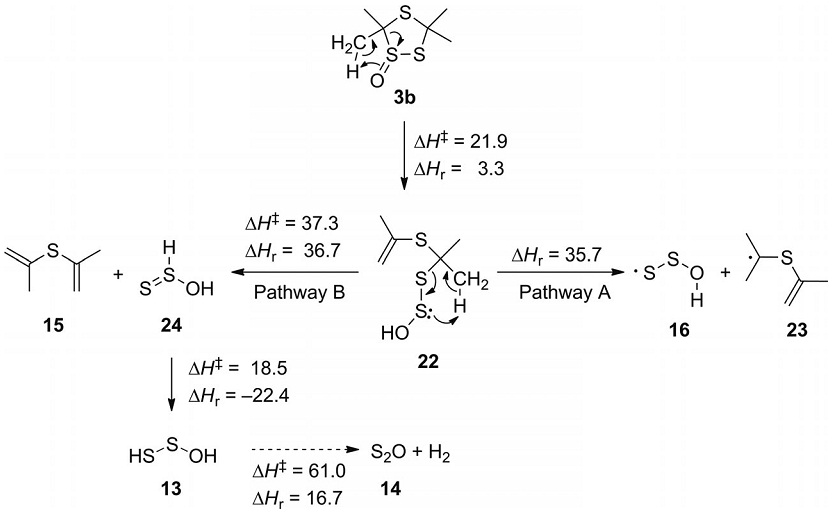
Flash vacuum pyrolysis of 3,3,5,5-tetramethyl-1,2,4-trithiolane 1-oxide performed at 700 °C yields the 1-oxatrisulfan-3-yl radical (HOSS·) along with disulfur monoxide (S2O) and diisopropyl sulfide, which were isolated in argon matrices at 10 K. Upon irradiation with UV light, the 1-oxatrisulfan-3-yl radical undergoes isomerization to the 1-oxatrisulfan-1-yl radical (HSSO·). Both radicals were identified by comparison of their computed and experimental IR and UV/Vis spectra. In addition, density functional theory (DFT) computations offer a plausible explanation of the most likely reaction mechanism, suggesting that the initial step is a 1,3-H shift with simultaneous ring opening. A 1-oxatrisulfane derivative formed thereby undergoes fragmentations via a radical and a competitive concerted pathway leading to the observed final products. The same mechanism also governs the thermal fragmentation of di-tert-butyl disulfide S-oxide. Its pyrolysis at 700 °C affords an analogous set of products, including the 1-oxatrisulfan-3-yl radical (HOSS·) as the key intermediate.
Conformations and Reactions of Bicyclo[3.2.1]oc-6-en-8-ylidene. Udo H. Brinker, Alexander A. Bespokoev, Hans Peter Reisenauer, and Peter R. Schreiner
J. Org. Chem. 2012, 77, 3800–3807. DOI: 10.1021/jo3001035
![Conformations and Reactions of Bicyclo[3.2.1]oct-6-en-8-ylidene](https://www.uni-giessen.de/de/fbz/fb08/Inst/organische-chemie/agschreiner/research/images/carbenes/ylideneudo/@@images/image-200-57e8ec5254966ff7a6a50994f41d253f.jpeg)
Oxathiirane. Peter R. Schreiner, Hans Peter Reisenauer, Jaroslaw Romanski, and Grzegorz Mloston J. Am. Chem. Soc. 2010, 132, 7240–7241. Download
|
We describe the first preparation of the long sought after parent oxathiirane from sulfine through photochemical rearrangement with light at 313 ± 10 nm in an Ar-matrix at 11 K. Oxathiirane was characterized by the extraordinarily good agreement of experimentally measured and CCSD(T)/cc-pVTZ (unscaled) computed vibrational frequencies both for the perhydrogenated and perdeuterated species. The title molecule is about 10 kcal mol–1 less stable than sulfine, in marked contrast to the isomer energy difference of dioxirane vs. carbonyl oxide (ca. –25 kcal mol–1). This is due to the strong positive polarization (blue potential) vs. the highly electronegative oxygen atom (red). The stability ordering and the relative energy differences of carbonyl vs. thiocarbonyl groups underlines the likely role oxathiiranes play in sulfur transfer reactions. |
 |
Thermal Reactions of Regioisomeric 1,2,4-Trithiolane S-Oxides.
Grzegorz Mloston, Jaroslaw Romanski, Michael L. McKee, Hans-Peter Reisenauer, and Peter R. Schreiner
Eur. J. Org. Chem. 2010, 2132–2137. Download
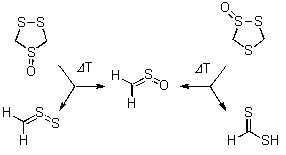
The products of the gas phase pyrolysis of two regioisomeric 1,2,4-trithiolane S-oxides were collected in an argon matrix at 10 K and studied by means of spectroscopic as well as computational methods. Whereas the main products of the pyrolysis of the ‘symmetrical’ S-oxide were identified as thioformaldehyde S-oxide and thioformaldehyde S-sulfide, the ‘non-symmetrical’ S-oxide gave predominantly dithioformic acid, which exists as a mixture of s-cis and s-trans conformers. We present a rationalization of the reaction pathways including density functional theory computations.
A formal carbon-sulfur triple bond: HCSOH. Peter R. Schreiner, Hans Peter Reisenauer, Jaroslaw Romanski, and Grzegorz Mloston Angew. Chem. Int. Ed. 2009, 48, 8133–8136. Download; Highlight: Neil Withers Nature Chem. 2009. Link; Robert Berger Nachr. Chem. 2010, 58, 7. Blog
|
Extremely rare: a CS triple bond can be assigned to HCSOH, a new molecule prepared through a photochemical [1,3]H-shift of sulfine H2C=S=O. But does this formal description agree with analyses on the basis of IR vibrations, bond lengths, bond orders, molecular orbitals, and compliance constants? Such types of molecules challenge and refine our current understanding of chemical bonding. |
 |
|
|
Infrared Signatures of the NCCO Radical. Peter R. Schreiner, Hans Peter Reisenauer, Edit Mátyus, Attila G. Császár, Ali Siddiqi, Andrew C. Simmonett, and Wesley D. Allen Phys. Chem. Chem. Phys. 2009, 11, 10385-10390. Download |
|
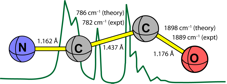
The first definitive infrared signatures of the elusive NCCO radical have been measured using a microwave discharge technique combined with low-temperature matrix-isolation spectroscopy, resulting in a consistent set of vibrational assignments for six isotopologues. The infrared spectra of these NCCO isotopologues were concomitantly established by rigorous variational nuclear-motion computations based on a high-level coupled-cluster quartic vibrational force field [ROCCSD(T)/cc-pCVQZ] and cubic dipole field [ROCCSD/cc-pCVTZ]. Our experimental and theoretical results for NCCO overturn the vibrational assignments in a NIST-JANAF compilation and those from a recent two-dimensional cross-spectral correlation analysis. For the parent isotopologue at 11 K in a nitrogen matrix, we find the signature bands n2(CO str.) = 1889.2 cm-1 and n3(CC str.) = 782.0 cm-1. Our variational vibrational computations reveal strong mixing of the n3 stretching fundamental and the n4+n5 bending combination level for all isotopologues. These Fermi resonances manifest a clear breakdown of the simple normal-mode picture of molecular vibrations at low energies. |
Prototypical Triplet Alkyl Phosphonatocarbenes.
Adelina Nemirowski, Hans Peter Reisenauer, Jaroslaw Romanski, Grzegorz Mloston, and Peter R. Schreiner,
J. Phys. Chem. A, 2008, 50, 13244. Download
|
Abstract. The current case study focuses on the generation, identification, and characterization of two representative mono- and disubstituted alkyl phosphonatocarbenes by means of matrix isolation techniques in conjunction with density functional theory [B3LYP/6-311++G(d,p)] and coupled cluster [CCSD(T)/cc-pVXZ, X = D, T] computations. The EPR measurements identify both carbenes as triplet ground-state species with D values of 0.660 and 0.623 cm–1 respectively, exhibiting persistency toward intramolecular reactions (the EPR signal observable in perfluoromethylcyclohexane up to around 70 K for the disubstituted molecule). While the reaction of the carbene center of the conformationally rich tetramethyl bisphosphonatocarbene with the CH bonds of the methyl groups leads to phosphaoxetane at room temperature, its fragmentation via a Wittig-type reaction during high vacuum flash pyrolysis (HVFP) results in dimethyl vinylphosphonate and methyl metaphosphate. The latter has been observed for the first time as an isolated entity. |
 |
Electronic Stabilization of Ground State Triplet Carbenes. Adelina Nemirowski and Peter R. Schreiner, J. Org. Chem. 2007, 72, 9533. Download
Abstract. Based on systematic ab initio (CCSD(T)/cc-pVDZ) studies of substituent effects, we present a concept for the construction of electronically stabilized triplet ground state carbenes with singlet−triplet energy separations (∆EST) exceeding that of methylene. Sterically demanding and conjugating substituents were excluded from the selection of model compounds under investigation, as these either destabilize both the singlet and the triplet states or delocalize unpaired spins away from the carbene carbon. Negative partial charges on the carbene center allow for the prediction of the electronic stabilization of substituted carbenes. To decrease carbene reactivity, we chose b-substituents with strong polar bonds. Among them, highly electronegative elements such as fluorine and oxygen enlarge the ∆EST value with respect to hydrogen, while chlorine does not due to p-orbital participation.
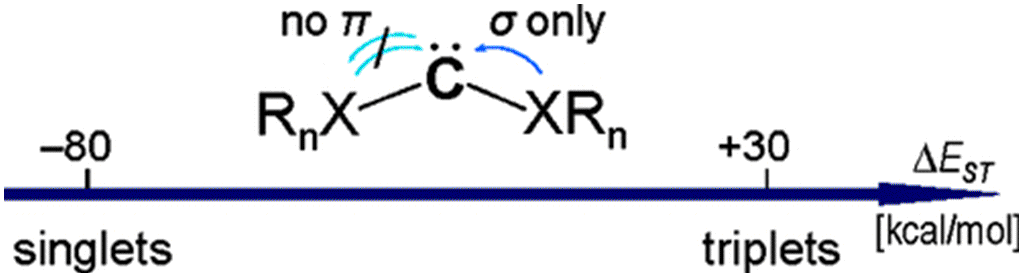
Dimethoxycarbene: Conformational Analysis of a Reactive Intermediate.
Hans Peter Reisenauer, Jaroslaw Romanski, Grzegorz Mloston, and Peter R. Schreiner,
Eur. J. Org. Chem. 2006, 4813. Download
Abstract. Dimethoxycarbene was prepared from an oxadiazoline precursor under high-vacuum flash pyrolysis (HVFP) conditions and was trapped at low temperatures by matrix isolation techniques (Ar, 10 K). The excellent agreement between the computed [CCSD(T)/cc-pVDZ] IR spectrum of the mixture of conformers of dimethoxycarbene and the experimentally measured IR absorptions allows a detailed analysis ofthe conformational preference of dimethoxycarbene. ItsUV spectrum is in agreement with earlier studies and our TD-B3LYP/6-311+G(d,p) computations. The computed [CCSD(T)/cc-pVDZ] rotational profile is rather steep and separates the s-trans,s-trans and s-cis,s-trans conformers by a 16 kcal mol-1 barrier, whilst the s-cis,s-cis conformer is too high-lying to be observable (+17 kcal mol-1). In marked contrast with the gauche,gauche minimum of dimethoxymethane, the s-trans,s-trans conformer of dimethoxycarbene is slightly preferred (0.5 kcal mol-1). The s-cis,s-trans conformer equilibrates at the high temperatures required during HFVP generation and both conformers can be identified in the IR spectrum of the argon matrix at 10 K. The conformational preference is partly due to the minimization of the overall dipole moment in the s-trans,s-trans conformer.
H–C–SiH3: Direct Generation and Spectroscopic Identification of Ethylidene’s Cousin. Peter R. Schreiner, Hans Peter Reisenauer, Kurt W. Sattelmeyer, and Wesley D. Allen,
J. Am. Chem. Soc. 2005, 127, 12156. Download
|
Abstract. Ground-state triplet silaethylidene, generated directly by the reaction of 3P carbon atoms with silane under matrix isolation conditions in solid Ar (10–12 K), has been thoroughly characterized by the EPR and IR spectra of both the parent and perdeuterated isotopologs. A theoretical anharmonic vibrational analysis based on a CCSD(T)/cc-pVTZ complete quartic force field gave remarkable agreement with the experimental IR fundamentals, generally within 10 cm–1 and without any empirical scaling of the ab initio frequencies. Silaethylidene exhibits a CS minimum with a H–C–Si angle near 153 °, but the barrier to H–C–Si linearity (C3v symmetry) is only 0.24 kcal mol–1. This minuscule barrier can be surmounted by zero-point vibrations, as evident from the EPR data. The triplet stabilizing effect of the electropositive SiH3 group amounts to about 15 kcal mol–1. |
|
Reactive Intermediates
Singlet carbenes incorporating a divalent carbon atom (R–C–R’) have grown from laboratory curiosities and theoreticians’ pet peeves into reagents in the growing field of stable carbene chemistry. Still, the experimental characterization of many simple yet fundamentally important carbenes (e.g., hydroxycarbenes, alkyl carbenes, etc.) is hampered by their high reactivity or lack of precursors. Hydroxycarbenes have been an unknown class of compounds until 2008, when our group reported the synthesis and characterization of hydroxymethylene (H–C–OH), whose preparation has been challenging organic chemists for more than 80 years. The reaction of hydroxycarbene with formaldehyde would be a source of simple sugars (the so-called “formose reaction” in the origin of life theory). Considerable efforts are ongoing to understand the formation and distribution of simple organics in extraterrestrial environments, and the examination of the structures and reactivities of prototypes such as hydroxycarbene may also provide glimpses of the prebiotic earth.
- Director: Univ.-Prof. Peter R. Schreiner
-
Co-workers:
- Reactive Intermediates
-
Sie entstammt dem zweiten großen mitteleuropäischen Gründungszeitalter, dem nachreformatorisch-konfessionellen, das von der 1527 errichteten Universität Marburg eingeleitet worden ist. Zuvor waren hessische Studenten vor allem nach Erfurt (seit 1392) und vorher nach Prag (seit 1348), Frankreich und Italien gezogen. Landgraf Ludwig V. von Hessen-Darmstadt schuf eine Universität im zweiten, nördlichen Zentrum seines kleinen Landes, 1607 in Gießen, als weiterhin lutherische Anstalt, weil Marburg nach der Teilung Hessens kalvinistisch geworden war. Im leidvollen Auf und Ab des Dreißigjährigen Krieges kam es zur Suspension der Ludoviciana zugunsten des alten Standorts (1624/1625). Der Westfälische Friede führte zur Wiederherstellung der Universität in Gießen (1650).
Reactive Intermediates
Singlet carbenes incorporating a divalent carbon atom (R–C–R’) have grown from laboratory curiosities and theoreticians’ pet peeves into reagents in the growing field of stable carbene chemistry. Still, the experimental characterization of many simple yet fundamentally important carbenes (e.g., hydroxycarbenes, alkyl carbenes, etc.) is hampered by their high reactivity or lack of precursors. Hydroxycarbenes have been an unknown class of compounds until 2008, when our group reported the synthesis and characterization of hydroxymethylene (H–C–OH), whose preparation has been challenging organic chemists for more than 80 years. The reaction of hydroxycarbene with formaldehyde would be a source of simple sugars (the so-called “formose reaction” in the origin of life theory). Considerable efforts are ongoing to understand the formation and distribution of simple organics in extraterrestrial environments, and the examination of the structures and reactivities of prototypes such as hydroxycarbene may also provide glimpses of the prebiotic earth.
- Director: Univ.-Prof. Peter R. Schreiner
-
Co-workers:
Gas phase preparation of carbonic acid and its monomethyl ester.
Hans Peter Reisenauer, Jan Philipp Wagner, Peter R. Schreiner
Angew. Chem. Int. Ed. 2014, 53, 11766–11771 (“hot paper”). DOI: 10.1002/ange.201406969.
Highlights:
a) Front cover of this issue;
b) Perspective: Götz Bucher and Wolfram Sander Science 2014, 346, 544–545; c) Feature: Carbonic Acid Crystal Forms Identified. Jyllian N. Kemsley C & EN News 2014, 92 (41), 28–29;
d)ChemistryViews: Carbonic Acid – And Yet It Exists!;
e) Innovations Report: Carbonic Acid—And Yet It Exists!;
f) Physorg.com: Preparation and characterization of gas-phase carbonic acid and its monomethyl ester.
Matrix Isolation and Spectroscopic Properties of the Methylsulfinyl Radical CH3(O)S•
Hans Peter Reisenauer,* Jaroslav Romanski, Grzegorz Mloston,* and Peter R. Schreiner,
Chem. Commun. 2013, 9467–9469. DOI: 10.1039/c3cc45379k

Thermolysis of 3,3,5,5-Tetramethyl-1,2,4-triothiolane-1-oxide: Matrix Isolation of the HOSS•-Radical.
Hans-Peter Reisenauer,* Grzegorz Mloston,* Jaroslaw Romanski, and Peter R. Schreiner,
Eur. J. Org. Chem. 2012, 3408–3415. DOI: 10.1002/ejoc.201200146

Flash vacuum pyrolysis of 3,3,5,5-tetramethyl-1,2,4-trithiolane 1-oxide performed at 700 °C yields the 1-oxatrisulfan-3-yl radical (HOSS·) along with disulfur monoxide (S2O) and diisopropyl sulfide, which were isolated in argon matrices at 10 K. Upon irradiation with UV light, the 1-oxatrisulfan-3-yl radical undergoes isomerization to the 1-oxatrisulfan-1-yl radical (HSSO·). Both radicals were identified by comparison of their computed and experimental IR and UV/Vis spectra. In addition, density functional theory (DFT) computations offer a plausible explanation of the most likely reaction mechanism, suggesting that the initial step is a 1,3-H shift with simultaneous ring opening. A 1-oxatrisulfane derivative formed thereby undergoes fragmentations via a radical and a competitive concerted pathway leading to the observed final products. The same mechanism also governs the thermal fragmentation of di-tert-butyl disulfide S-oxide. Its pyrolysis at 700 °C affords an analogous set of products, including the 1-oxatrisulfan-3-yl radical (HOSS·) as the key intermediate.
Conformations and Reactions of Bicyclo[3.2.1]oc-6-en-8-ylidene.
Udo H. Brinker, Alexander A. Bespokoev, Hans Peter Reisenauer, and Peter R. Schreiner
J. Org. Chem. 2012, 77, 3800–3807. DOI: 10.1021/jo3001035
![Conformations and Reactions of Bicyclo[3.2.1]oct-6-en-8-ylidene Conformations and Reactions of Bicyclo[3.2.1]oct-6-en-8-ylidene](../images/carbenes/ylideneudo/@@images/14a29e16-fdfd-4a46-8915-402aeb98f869.jpeg) Bicyclo[3.2.1]oct-6-en-8-ylidene (1) can assume either the conformation of “classical” carbene 1a or that of foiled carbene 1b in which the divalent carbon bends toward the double bond. Oxadiazoline precursors for the generation of 1 were prepared, followed by photochemical and thermal decomposition as well as flash vacuum pyrolysis (FVP) of a tosyl hydrazone sodium salt precursor, to give a number of rearrangement products. Matrix isolation experiments demonstrate the presence of a diazo intermediate and methyl acetate in all photochemical and thermal precursor reactions. The major product from rearrangements of “classical” bridged carbene 1a is bicyclo[3.3.0]octa-1,3-diene as a result of an alkyl shift, while dihydrosemibullvalene formed from a 1,3-C–H insertion. In contrast, thus far unknown strained bicyclo[4.2.0]octa-1,7-diene formed by a vinyl shift in foiled carbene 1b. The experimental results are corroborated by density functional theory (DFT), MP2, and G4 computations.
Bicyclo[3.2.1]oct-6-en-8-ylidene (1) can assume either the conformation of “classical” carbene 1a or that of foiled carbene 1b in which the divalent carbon bends toward the double bond. Oxadiazoline precursors for the generation of 1 were prepared, followed by photochemical and thermal decomposition as well as flash vacuum pyrolysis (FVP) of a tosyl hydrazone sodium salt precursor, to give a number of rearrangement products. Matrix isolation experiments demonstrate the presence of a diazo intermediate and methyl acetate in all photochemical and thermal precursor reactions. The major product from rearrangements of “classical” bridged carbene 1a is bicyclo[3.3.0]octa-1,3-diene as a result of an alkyl shift, while dihydrosemibullvalene formed from a 1,3-C–H insertion. In contrast, thus far unknown strained bicyclo[4.2.0]octa-1,7-diene formed by a vinyl shift in foiled carbene 1b. The experimental results are corroborated by density functional theory (DFT), MP2, and G4 computations.
Oxathiirane.
Peter R. Schreiner, Hans Peter Reisenauer, Jaroslaw Romanski, and Grzegorz Mloston
J. Am. Chem. Soc. 2010, 132, 7240–7241. Download
|
We describe the first preparation of the long sought after parent oxathiirane from sulfine through photochemical rearrangement with light at 313 ± 10 nm in an Ar-matrix at 11 K. Oxathiirane was characterized by the extraordinarily good agreement of experimentally measured and CCSD(T)/cc-pVTZ (unscaled) computed vibrational frequencies both for the perhydrogenated and perdeuterated species. The title molecule is about 10 kcal mol–1 less stable than sulfine, in marked contrast to the isomer energy difference of dioxirane vs. carbonyl oxide (ca. –25 kcal mol–1). This is due to the strong positive polarization (blue potential) vs. the highly electronegative oxygen atom (red). The stability ordering and the relative energy differences of carbonyl vs. thiocarbonyl groups underlines the likely role oxathiiranes play in sulfur transfer reactions. |
 |
Thermal Reactions of Regioisomeric 1,2,4-Trithiolane S-Oxides.
Grzegorz Mloston, Jaroslaw Romanski, Michael L. McKee, Hans-Peter Reisenauer, and Peter R. Schreiner
Eur. J. Org. Chem. 2010, 2132–2137. Download

The products of the gas phase pyrolysis of two regioisomeric 1,2,4-trithiolane S-oxides were collected in an argon matrix at 10 K and studied by means of spectroscopic as well as computational methods. Whereas the main products of the pyrolysis of the ‘symmetrical’ S-oxide were identified as thioformaldehyde S-oxide and thioformaldehyde S-sulfide, the ‘non-symmetrical’ S-oxide gave predominantly dithioformic acid, which exists as a mixture of s-cis and s-trans conformers. We present a rationalization of the reaction pathways including density functional theory computations.
A formal carbon-sulfur triple bond: HCSOH.
Peter R. Schreiner, Hans Peter Reisenauer, Jaroslaw Romanski, and Grzegorz Mloston
Angew. Chem. Int. Ed. 2009, 48, 8133–8136. Download; Highlight: Neil Withers Nature Chem. 2009. Link; Robert Berger Nachr. Chem. 2010, 58, 7. Blog
|
Extremely rare: a CS triple bond can be assigned to HCSOH, a new molecule prepared through a photochemical [1,3]H-shift of sulfine H2C=S=O. But does this formal description agree with analyses on the basis of IR vibrations, bond lengths, bond orders, molecular orbitals, and compliance constants? Such types of molecules challenge and refine our current understanding of chemical bonding. |
 |
Infrared Signatures of the NCCO Radical.
|
|
Peter R. Schreiner, Hans Peter Reisenauer, Edit Mátyus, Attila G. Császár, Ali Siddiqi, Andrew C. Simmonett, and Wesley D. Allen Phys. Chem. Chem. Phys. 2009, 11, 10385-10390. Download |
|
The first definitive infrared signatures of the elusive NCCO radical have been measured using a microwave discharge technique combined with low-temperature matrix-isolation spectroscopy, resulting in a consistent set of vibrational assignments for six isotopologues. The infrared spectra of these NCCO isotopologues were concomitantly established by rigorous variational nuclear-motion computations based on a high-level coupled-cluster quartic vibrational force field [ROCCSD(T)/cc-pCVQZ] and cubic dipole field [ROCCSD/cc-pCVTZ]. Our experimental and theoretical results for NCCO overturn the vibrational assignments in a NIST-JANAF compilation and those from a recent two-dimensional cross-spectral correlation analysis. For the parent isotopologue at 11 K in a nitrogen matrix, we find the signature bands n2(CO str.) = 1889.2 cm-1 and n3(CC str.) = 782.0 cm-1. Our variational vibrational computations reveal strong mixing of the n3 stretching fundamental and the n4+n5 bending combination level for all isotopologues. These Fermi resonances manifest a clear breakdown of the simple normal-mode picture of molecular vibrations at low energies. |
Prototypical Triplet Alkyl Phosphonatocarbenes.
Adelina Nemirowski, Hans Peter Reisenauer, Jaroslaw Romanski, Grzegorz Mloston, and Peter R. Schreiner,
J. Phys. Chem. A, 2008, 50, 13244. Download
|
Abstract. The current case study focuses on the generation, identification, and characterization of two representative mono- and disubstituted alkyl phosphonatocarbenes by means of matrix isolation techniques in conjunction with density functional theory [B3LYP/6-311++G(d,p)] and coupled cluster [CCSD(T)/cc-pVXZ, X = D, T] computations. The EPR measurements identify both carbenes as triplet ground-state species with D values of 0.660 and 0.623 cm–1 respectively, exhibiting persistency toward intramolecular reactions (the EPR signal observable in perfluoromethylcyclohexane up to around 70 K for the disubstituted molecule). While the reaction of the carbene center of the conformationally rich tetramethyl bisphosphonatocarbene with the CH bonds of the methyl groups leads to phosphaoxetane at room temperature, its fragmentation via a Wittig-type reaction during high vacuum flash pyrolysis (HVFP) results in dimethyl vinylphosphonate and methyl metaphosphate. The latter has been observed for the first time as an isolated entity. |
 |
Electronic Stabilization of Ground State Triplet Carbenes.
Adelina Nemirowski and Peter R. Schreiner, J. Org. Chem. 2007, 72, 9533. Download
Abstract. Based on systematic ab initio (CCSD(T)/cc-pVDZ) studies of substituent effects, we present a concept for the construction of electronically stabilized triplet ground state carbenes with singlet−triplet energy separations (∆EST) exceeding that of methylene. Sterically demanding and conjugating substituents were excluded from the selection of model compounds under investigation, as these either destabilize both the singlet and the triplet states or delocalize unpaired spins away from the carbene carbon. Negative partial charges on the carbene center allow for the prediction of the electronic stabilization of substituted carbenes. To decrease carbene reactivity, we chose b-substituents with strong polar bonds. Among them, highly electronegative elements such as fluorine and oxygen enlarge the ∆EST value with respect to hydrogen, while chlorine does not due to p-orbital participation.

Dimethoxycarbene: Conformational Analysis of a Reactive Intermediate.
Hans Peter Reisenauer, Jaroslaw Romanski, Grzegorz Mloston, and Peter R. Schreiner,
Eur. J. Org. Chem. 2006, 4813. Download
Abstract. Dimethoxycarbene was prepared from an oxadiazoline precursor under high-vacuum flash pyrolysis (HVFP) conditions and was trapped at low temperatures by matrix isolation techniques (Ar, 10 K). The excellent agreement between the computed [CCSD(T)/cc-pVDZ] IR spectrum of the mixture of conformers of dimethoxycarbene and the experimentally measured IR absorptions allows a detailed analysis ofthe conformational preference of dimethoxycarbene. ItsUV spectrum is in agreement with earlier studies and our TD-B3LYP/6-311+G(d,p) computations. The computed [CCSD(T)/cc-pVDZ] rotational profile is rather steep and separates the s-trans,s-trans and s-cis,s-trans conformers by a 16 kcal mol-1 barrier, whilst the s-cis,s-cis conformer is too high-lying to be observable (+17 kcal mol-1). In marked contrast with the gauche,gauche minimum of dimethoxymethane, the s-trans,s-trans conformer of dimethoxycarbene is slightly preferred (0.5 kcal mol-1). The s-cis,s-trans conformer equilibrates at the high temperatures required during HFVP generation and both conformers can be identified in the IR spectrum of the argon matrix at 10 K. The conformational preference is partly due to the minimization of the overall dipole moment in the s-trans,s-trans conformer.
H–C–SiH3: Direct Generation and Spectroscopic Identification of Ethylidene’s Cousin.
Peter R. Schreiner, Hans Peter Reisenauer, Kurt W. Sattelmeyer, and Wesley D. Allen,
J. Am. Chem. Soc. 2005, 127, 12156. Download
|
Abstract. Ground-state triplet silaethylidene, generated directly by the reaction of 3P carbon atoms with silane under matrix isolation conditions in solid Ar (10–12 K), has been thoroughly characterized by the EPR and IR spectra of both the parent and perdeuterated isotopologs. A theoretical anharmonic vibrational analysis based on a CCSD(T)/cc-pVTZ complete quartic force field gave remarkable agreement with the experimental IR fundamentals, generally within 10 cm–1 and without any empirical scaling of the ab initio frequencies. Silaethylidene exhibits a CS minimum with a H–C–Si angle near 153 °, but the barrier to H–C–Si linearity (C3v symmetry) is only 0.24 kcal mol–1. This minuscule barrier can be surmounted by zero-point vibrations, as evident from the EPR data. The triplet stabilizing effect of the electropositive SiH3 group amounts to about 15 kcal mol–1. |
|